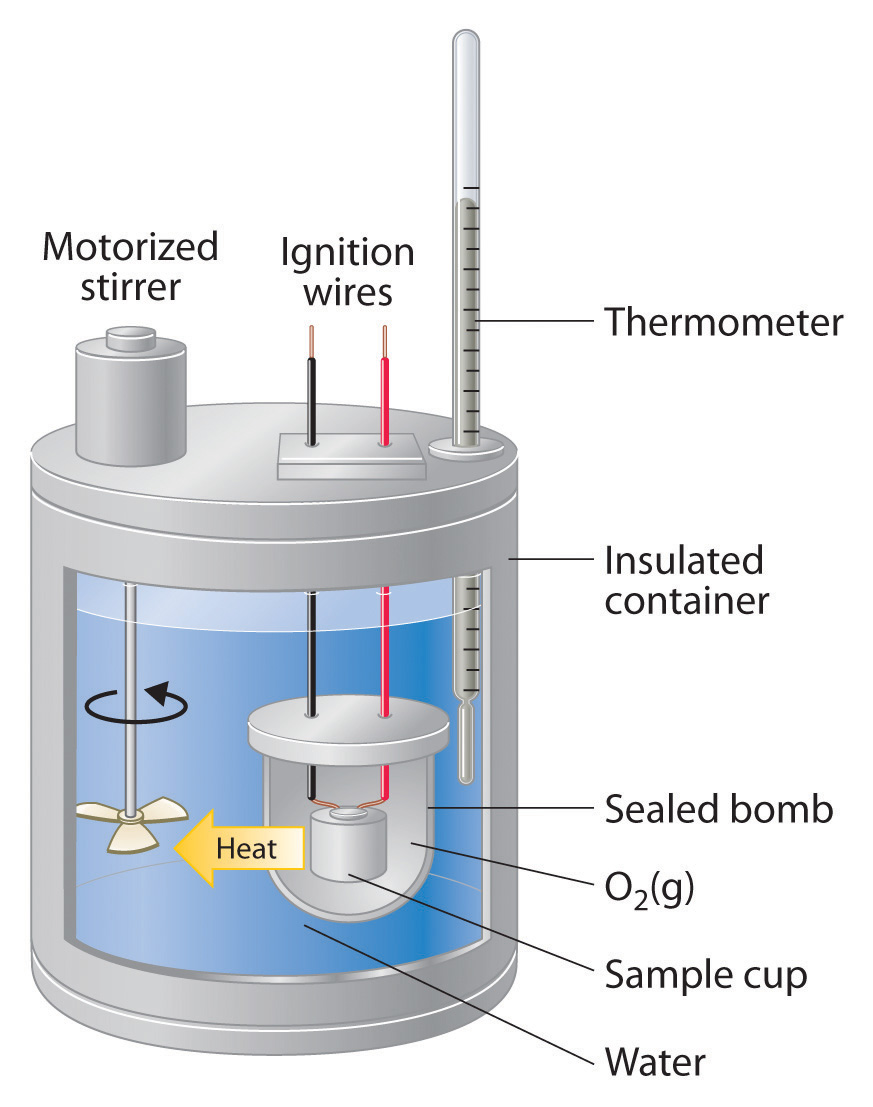
Hypothesis For Calorimetry And Specific Heat Lab . the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature of the sample changes as it either. • perform calculations involving change in temperature, specific heat capacity, and heat. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. • use scientific reasoning skills. Heat, specific heat, calorie, temperature. calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. On completion of this experiment, students should be able.
from exodnulby.blob.core.windows.net
• perform calculations involving change in temperature, specific heat capacity, and heat. specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature of the sample changes as it either. On completion of this experiment, students should be able. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. Heat, specific heat, calorie, temperature. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. • use scientific reasoning skills.
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog
Hypothesis For Calorimetry And Specific Heat Lab • perform calculations involving change in temperature, specific heat capacity, and heat. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. On completion of this experiment, students should be able. • use scientific reasoning skills. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. Heat, specific heat, calorie, temperature. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature of the sample changes as it either. • perform calculations involving change in temperature, specific heat capacity, and heat.
From www.coursehero.com
[Solved] Lab 6 Specific Heats and Calorimetry General Chemistry I Hypothesis For Calorimetry And Specific Heat Lab the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. On completion of this experiment, students should be able. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. first, the. Hypothesis For Calorimetry And Specific Heat Lab.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Hypothesis For Calorimetry And Specific Heat Lab the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. first, the specific heat (s) of a substance is the amount. Hypothesis For Calorimetry And Specific Heat Lab.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity Hypothesis For Calorimetry And Specific Heat Lab On completion of this experiment, students should be able. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature of the sample changes as. Hypothesis For Calorimetry And Specific Heat Lab.
From www.qwivy.com
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. specific heat is an intensive property of a single phase (solid, liquid or gas). Hypothesis For Calorimetry And Specific Heat Lab.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Hypothesis For Calorimetry And Specific Heat Lab Heat, specific heat, calorie, temperature. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. On completion of this experiment, students should be able. • use scientific reasoning skills. specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Hypothesis For Calorimetry And Specific Heat Lab.
From www.chegg.com
Solved Lab 5 Report Calorimetry Determination of Specific Hypothesis For Calorimetry And Specific Heat Lab Heat, specific heat, calorie, temperature. On completion of this experiment, students should be able. • use scientific reasoning skills. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. calorimetry will be used to investigate specific heats of materials and the latent heat associated. Hypothesis For Calorimetry And Specific Heat Lab.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Hypothesis For Calorimetry And Specific Heat Lab • use scientific reasoning skills. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. specific heat is an intensive property. Hypothesis For Calorimetry And Specific Heat Lab.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. Heat, specific heat, calorie, temperature. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. On completion of this experiment, students should be able. . Hypothesis For Calorimetry And Specific Heat Lab.
From www.slidemake.com
Einstein Theory Of Specific Heat Presentation Hypothesis For Calorimetry And Specific Heat Lab specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature of the sample changes as it either. On completion of this experiment, students should be able. Heat, specific heat, calorie, temperature. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir. Hypothesis For Calorimetry And Specific Heat Lab.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Curtin Hypothesis For Calorimetry And Specific Heat Lab Heat, specific heat, calorie, temperature. On completion of this experiment, students should be able. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. • use scientific reasoning skills. • perform calculations involving change in temperature, specific heat capacity, and heat. specific heat. Hypothesis For Calorimetry And Specific Heat Lab.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. • perform calculations involving change in temperature, specific heat capacity, and heat. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. specific. Hypothesis For Calorimetry And Specific Heat Lab.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Hypothesis For Calorimetry And Specific Heat Lab the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. • use scientific reasoning skills. On completion of this experiment, students should be able. specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Hypothesis For Calorimetry And Specific Heat Lab.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Hypothesis For Calorimetry And Specific Heat Lab • perform calculations involving change in temperature, specific heat capacity, and heat. specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature of the sample changes as it either. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature. Hypothesis For Calorimetry And Specific Heat Lab.
From www.studocu.com
Calorimetry and Heat Transfer Calorimetry and Heat Transfer Objective Hypothesis For Calorimetry And Specific Heat Lab first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. • perform calculations involving change in temperature, specific heat. Hypothesis For Calorimetry And Specific Heat Lab.
From www.scribd.com
lab 4 calorimetry lab Temperature Heat Capacity Hypothesis For Calorimetry And Specific Heat Lab On completion of this experiment, students should be able. • use scientific reasoning skills. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. • perform calculations involving change in temperature, specific heat capacity, and heat. calorimetry will be used to investigate specific. Hypothesis For Calorimetry And Specific Heat Lab.
From www.chegg.com
Solved REPORT FOR EXPERIMENT5 Calorimetry and Specific Heat Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Hypothesis For Calorimetry And Specific Heat Lab.
From slidetodoc.com
Thermodynamics theory Specific Heat Internal Energy Hypothesis For Calorimetry And Specific Heat Lab • perform calculations involving change in temperature, specific heat capacity, and heat. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. Heat, specific heat, calorie, temperature. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid. Hypothesis For Calorimetry And Specific Heat Lab.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Hypothesis For Calorimetry And Specific Heat Lab specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature of the sample changes as it either. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. the amount of heat absorbed or. Hypothesis For Calorimetry And Specific Heat Lab.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. On completion of this experiment, students should be able. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. • use scientific reasoning skills. . Hypothesis For Calorimetry And Specific Heat Lab.
From www.chegg.com
Solved Data Table for the Specific Heat and Calorimeter Lab Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. Heat, specific heat, calorie, temperature. • perform calculations involving change in temperature, specific heat. Hypothesis For Calorimetry And Specific Heat Lab.
From www.studocu.com
Copy of Lab 5 Calorimetry and Specific Heat Lab Calorimetry and Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. • use scientific reasoning skills. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. On completion of this experiment, students should be able. . Hypothesis For Calorimetry And Specific Heat Lab.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Hypothesis For Calorimetry And Specific Heat Lab On completion of this experiment, students should be able. • perform calculations involving change in temperature, specific heat capacity, and heat. • use scientific reasoning skills. Heat, specific heat, calorie, temperature. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. calorimetry will. Hypothesis For Calorimetry And Specific Heat Lab.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. • use scientific reasoning skills. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. • perform calculations involving change in temperature, specific heat capacity, and. Hypothesis For Calorimetry And Specific Heat Lab.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. • use scientific reasoning skills. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. Heat, specific heat, calorie, temperature. • perform calculations involving change in. Hypothesis For Calorimetry And Specific Heat Lab.
From studylib.net
Lab 11b Specific Heat & Heat Capacity Hypothesis For Calorimetry And Specific Heat Lab first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. Heat, specific heat, calorie, temperature. • perform calculations involving change in temperature, specific heat. Hypothesis For Calorimetry And Specific Heat Lab.
From www.scribd.com
Lab 07Specific Heat & Calorimetry PDF PDF Heat Temperature Hypothesis For Calorimetry And Specific Heat Lab • perform calculations involving change in temperature, specific heat capacity, and heat. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. • use scientific reasoning skills. calorimetry will be used to investigate specific heats of materials and the latent heat associated with. Hypothesis For Calorimetry And Specific Heat Lab.
From www.studocu.com
3210 07 04 student guide Copyright © Edgenuity Inc. Lab Calorimetry Hypothesis For Calorimetry And Specific Heat Lab specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature of the sample changes as it either. Heat, specific heat, calorie, temperature. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. the. Hypothesis For Calorimetry And Specific Heat Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Hypothesis For Calorimetry And Specific Heat Lab Heat, specific heat, calorie, temperature. On completion of this experiment, students should be able. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. . Hypothesis For Calorimetry And Specific Heat Lab.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Hypothesis For Calorimetry And Specific Heat Lab On completion of this experiment, students should be able. Heat, specific heat, calorie, temperature. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. • perform calculations involving change in temperature, specific heat capacity, and heat. the amount of heat absorbed or released. Hypothesis For Calorimetry And Specific Heat Lab.
From ar.inspiredpencil.com
Specific Heat Lab Calorimeter Hypothesis For Calorimetry And Specific Heat Lab calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. Heat, specific heat, calorie, temperature. the specific heat capacity (or just specific heat) is the heat. Hypothesis For Calorimetry And Specific Heat Lab.
From kaffee.50webs.com
Lab Calorimetry Hypothesis For Calorimetry And Specific Heat Lab first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. On completion of this experiment, students should be able. calorimetry will be used to investigate specific heats of materials and the latent heat associated with a phase change. • perform calculations involving change. Hypothesis For Calorimetry And Specific Heat Lab.
From studylib.net
Calorimetry Lab Specific Heat Capacity Hypothesis For Calorimetry And Specific Heat Lab • perform calculations involving change in temperature, specific heat capacity, and heat. Heat, specific heat, calorie, temperature. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. calorimetry will be used to investigate specific heats of materials and the latent heat associated with. Hypothesis For Calorimetry And Specific Heat Lab.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Hypothesis For Calorimetry And Specific Heat Lab On completion of this experiment, students should be able. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. • perform. Hypothesis For Calorimetry And Specific Heat Lab.
From users.highland.edu
Calorimetry Hypothesis For Calorimetry And Specific Heat Lab • perform calculations involving change in temperature, specific heat capacity, and heat. On completion of this experiment, students should be able. the specific heat capacity (or just specific heat) is the heat capacity per gram of the reservoir liquid and is given the symbol, c. specific heat is an intensive property of a single phase (solid, liquid. Hypothesis For Calorimetry And Specific Heat Lab.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Hypothesis For Calorimetry And Specific Heat Lab • perform calculations involving change in temperature, specific heat capacity, and heat. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature of the sample. Hypothesis For Calorimetry And Specific Heat Lab.