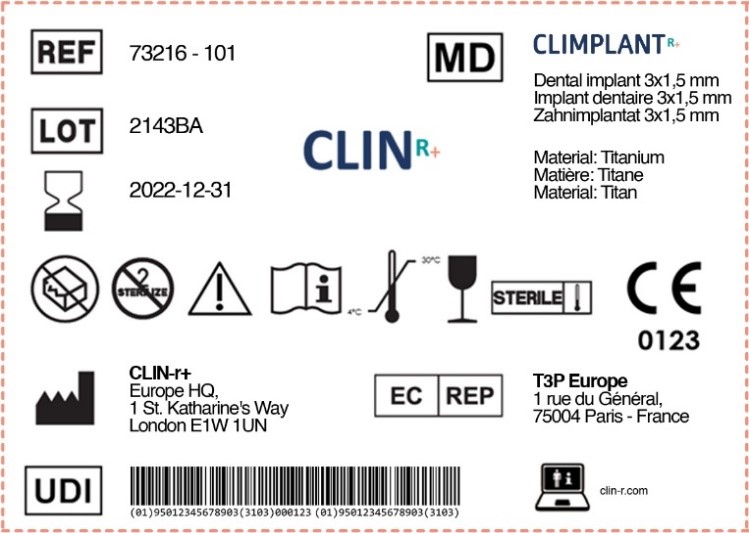
Fda Off-Label Use Medical Device . This term can mean that the drug is: Used for a disease or medical condition that it is not. In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. How does fda classify medical devices? Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices.
from www.vrogue.co
Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. This term can mean that the drug is: How does fda classify medical devices? Used for a disease or medical condition that it is not. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications.
Fda Medical Device Label Symbols vrogue.co
Fda Off-Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. How does fda classify medical devices? Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. This term can mean that the drug is: Used for a disease or medical condition that it is not.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Off-Label Use Medical Device Used for a disease or medical condition that it is not. Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The new rule and its accompanying. Fda Off-Label Use Medical Device.
From stellacenter.com
OffLabel Medical Treatments Proven Effective Stella Fda Off-Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. Used for. Fda Off-Label Use Medical Device.
From www.youtube.com
FDA's Role in the Off Label Use of Medical Devices Explained by Dr Fda Off-Label Use Medical Device This term can mean that the drug is: On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. How does fda classify medical devices? Good medical practice and the best interests of the patient. Fda Off-Label Use Medical Device.
From www.vrogue.co
Fda Launches New page To Promote Use Of Symbols In vrogue.co Fda Off-Label Use Medical Device This term can mean that the drug is: On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. How does fda classify medical devices? Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. Good medical practice and the best interests. Fda Off-Label Use Medical Device.
From mungfali.com
FDA Medical Device Label Symbols Fda Off-Label Use Medical Device Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. This term can mean that the drug is: How does fda classify medical devices? Used for a. Fda Off-Label Use Medical Device.
From www.pharmaceutical-technology.com
FDA cracks down on offlabel drug use messaging Pharmaceutical Technology Fda Off-Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. Used for a disease or medical condition that it is not. In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. How does fda classify medical devices? This term can mean that the drug is:. Fda Off-Label Use Medical Device.
From virtsugar.weebly.com
Medical device fda label expert witness cv virtsugar Fda Off-Label Use Medical Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Used for a disease or medical condition that it is not. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. How does fda classify medical devices? Once medical drugs and devices are approved. Fda Off-Label Use Medical Device.
From mungfali.com
Medical Device Labeling Symbols Fda Off-Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. How does fda classify medical devices? The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare. Fda Off-Label Use Medical Device.
From dandelionsandthings.blogspot.com
30 Medical Device Label Symbols Label Design Ideas 2020 Fda Off-Label Use Medical Device Used for a disease or medical condition that it is not. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. How does fda classify medical devices?. Fda Off-Label Use Medical Device.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Fda Off-Label Use Medical Device In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. This term can mean that the drug is: Once. Fda Off-Label Use Medical Device.
From www.pm360online.com
What Does the FDA’s New Offlabel Guidance Mean for Pharma? PM360 Fda Off-Label Use Medical Device In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. This term can mean that the drug is: Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. Once medical drugs and devices are approved for marketing by the fda they can legally be used. Fda Off-Label Use Medical Device.
From barcode-labels.com
Medical Device Labels Electronic Imaging Materials Fda Off-Label Use Medical Device Used for a disease or medical condition that it is not. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. How does fda classify medical devices? In accordance with. Fda Off-Label Use Medical Device.
From www.druganddevicelawblog.com
Examining The Amarin/FDA OffLabel Promotion Settlement Drug & Device Law Fda Off-Label Use Medical Device Used for a disease or medical condition that it is not. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. This term can mean that the drug is: On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. In accordance with the federal. Fda Off-Label Use Medical Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Off-Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and. Fda Off-Label Use Medical Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Off-Label Use Medical Device Used for a disease or medical condition that it is not. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. This term can mean that the drug is: On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. In accordance with the federal. Fda Off-Label Use Medical Device.
From www.medicaldesignandoutsourcing.com
FDA to clarify role of offlabel uses in medical device approvals Fda Off-Label Use Medical Device This term can mean that the drug is: In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. Used for a disease or medical condition that it is not. The new rule and its accompanying. Fda Off-Label Use Medical Device.
From www.nicelabel.com
FDA UDI compliant labelling NiceLabel Fda Off-Label Use Medical Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. This term can mean that the. Fda Off-Label Use Medical Device.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Off-Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. How does fda classify medical devices? Used for a disease or medical condition that it is not. On tuesday, october. Fda Off-Label Use Medical Device.
From emmainternational.com
Discovering FDALabel Your GoTo Labelling Tool Fda Off-Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. This term can mean that the drug is: The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge. Fda Off-Label Use Medical Device.
From www.northcarolinaproductliabilitylawyer.com
OffLabel Use Category Archives — North Carolina Product Liability Fda Off-Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. How does fda classify medical devices? Once medical drugs and devices are approved for marketing by the fda they can legally be used for. Fda Off-Label Use Medical Device.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Fda Off-Label Use Medical Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. This term can mean that the drug is: Used for a disease or medical condition that it is not. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. Once medical drugs and devices. Fda Off-Label Use Medical Device.
From www.audible.com
FDA's Role in the Off Label Use of Medical Devices Explained by Dr Fda Off-Label Use Medical Device How does fda classify medical devices? The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Once medical drugs and devices are approved for marketing by the fda they can legally be used for. Fda Off-Label Use Medical Device.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Fda Off-Label Use Medical Device Used for a disease or medical condition that it is not. How does fda classify medical devices? This term can mean that the drug is: Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. In accordance with the federal food, drug, and cosmetic act, fda places. Fda Off-Label Use Medical Device.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Fda Off-Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. This term can mean that the drug is: In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. Once medical drugs and devices are approved for marketing by the fda they can legally be used. Fda Off-Label Use Medical Device.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Fda Off-Label Use Medical Device Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. How does fda classify medical devices? The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. This term can mean that the drug is: Good medical practice. Fda Off-Label Use Medical Device.
From old.sermitsiaq.ag
Medical Device Label Template Fda Off-Label Use Medical Device How does fda classify medical devices? The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes. Fda Off-Label Use Medical Device.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Fda Off-Label Use Medical Device In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. Used for a disease or medical condition that it is not. How does fda classify medical devices? On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. This term can mean that the drug is: Good medical practice and. Fda Off-Label Use Medical Device.
From starboardmedical.com
Medical Devices and The Danger of Off Label Use Starboard Medical Fda Off-Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Used for a disease or medical condition that it is not. How does fda classify medical devices? Once medical drugs and devices are approved. Fda Off-Label Use Medical Device.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Off-Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. This term can mean that the drug is: The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Used for a disease or medical condition that it is not. In. Fda Off-Label Use Medical Device.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Off-Label Use Medical Device How does fda classify medical devices? Used for a disease or medical condition that it is not. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. On tuesday, october. Fda Off-Label Use Medical Device.
From www.pm360online.com
What Does the FDA’s New Offlabel Guidance Mean for Pharma? PM360 Fda Off-Label Use Medical Device Used for a disease or medical condition that it is not. This term can mean that the drug is: The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. Good medical practice and the best. Fda Off-Label Use Medical Device.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Off-Label Use Medical Device Once medical drugs and devices are approved for marketing by the fda they can legally be used for purposes and in ways other. This term can mean that the drug is: How does fda classify medical devices? Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. Used for a. Fda Off-Label Use Medical Device.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Off-Label Use Medical Device In accordance with the federal food, drug, and cosmetic act, fda places all medical devices. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Used for a disease or medical condition that it is not. Good medical practice and the best interests of the patient require that physicians use. Fda Off-Label Use Medical Device.
From www.youtube.com
Good OffLabel Use Practice of Medicines YouTube Fda Off-Label Use Medical Device How does fda classify medical devices? The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. Once medical drugs and devices are approved for marketing by the fda they can. Fda Off-Label Use Medical Device.
From studylib.net
OFFLABEL USE OF MEDICAL PRODUCTS WARRANTY AND Fda Off-Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. How does fda classify medical devices? The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Used for a disease or medical condition that it is not. On tuesday, october. Fda Off-Label Use Medical Device.