Magnesium And Potassium Nitrate Reaction . We described a precipitation reaction in which a colorless solution of. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. The potassium and nitrate ions. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. Magnesium is a more reactive metal than copper. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. If precipitation is expected, write a. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3].
from www.numerade.com
Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. The potassium and nitrate ions. If precipitation is expected, write a. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. We described a precipitation reaction in which a colorless solution of. K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. Magnesium is a more reactive metal than copper.
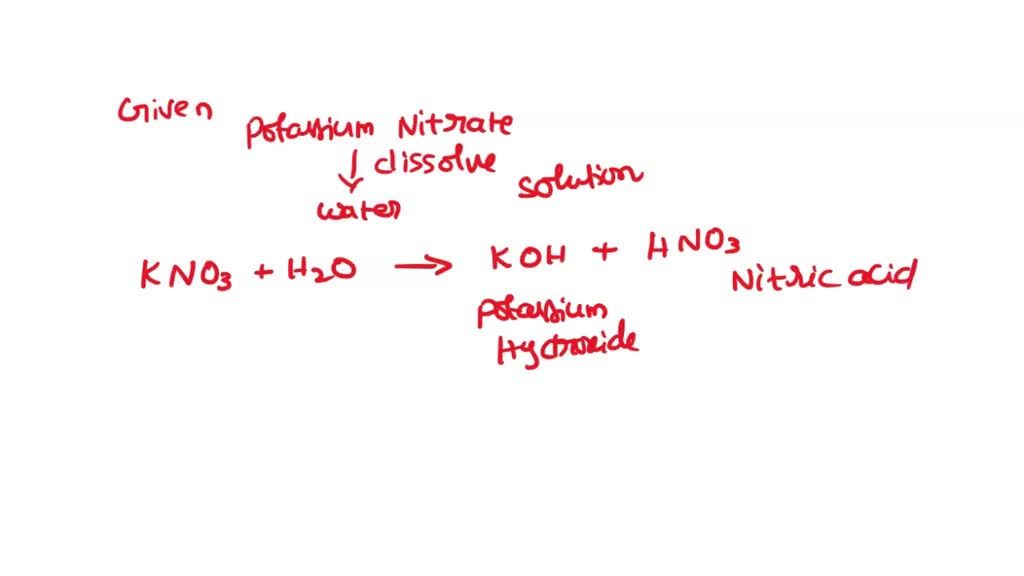
SOLVED 'QUESTION 9 potassium nitrate, KNO3 dissolves water, H2O, the
Magnesium And Potassium Nitrate Reaction Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. Magnesium is a more reactive metal than copper. We described a precipitation reaction in which a colorless solution of. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If precipitation is expected, write a. The potassium and nitrate ions.
From ar.inspiredpencil.com
Reactants In A Chemical Equation Magnesium And Potassium Nitrate Reaction Magnesium is a more reactive metal than copper. If precipitation is expected, write a. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. We described a precipitation reaction in which a colorless solution of. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Magnesium nitrate + potassium hydroxide. Magnesium And Potassium Nitrate Reaction.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Magnesium And Potassium Nitrate Reaction Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. Magnesium is a more reactive metal than copper. K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. Predict the result of mixing reasonably. Magnesium And Potassium Nitrate Reaction.
From www.youtube.com
Writing Ionic Formulas Magnesium Nitrate YouTube Magnesium And Potassium Nitrate Reaction K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. The potassium and nitrate ions. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. Magnesium is a. Magnesium And Potassium Nitrate Reaction.
From www.slideserve.com
PPT Types of Chemical Reactions Single and Double Displacement Magnesium And Potassium Nitrate Reaction The potassium and nitrate ions. We described a precipitation reaction in which a colorless solution of. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. Predict the result of mixing reasonably concentrated solutions of the following ionic. Magnesium And Potassium Nitrate Reaction.
From www.numerade.com
SOLVED 'QUESTION 9 potassium nitrate, KNO3 dissolves water, H2O, the Magnesium And Potassium Nitrate Reaction Magnesium is a more reactive metal than copper. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. If. Magnesium And Potassium Nitrate Reaction.
From www.numerade.com
SOLVED Write chemical equations for the following singlereplacement Magnesium And Potassium Nitrate Reaction Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it.. Magnesium And Potassium Nitrate Reaction.
From onlinelibrary.wiley.com
Functioning of potassium and magnesium in photosynthesis, photosynthate Magnesium And Potassium Nitrate Reaction Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. We described a precipitation reaction in which a colorless solution of. The potassium and nitrate ions. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. Magnesium. Magnesium And Potassium Nitrate Reaction.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Magnesium And Potassium Nitrate Reaction K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. A precipitation reaction is. Magnesium And Potassium Nitrate Reaction.
From www.nagwa.com
Question Video Ordering the Reactions of Magnesium Metal with Varying Magnesium And Potassium Nitrate Reaction When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. We described a precipitation reaction in which a colorless solution of. Magnesium is a more reactive metal than copper. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. The potassium. Magnesium And Potassium Nitrate Reaction.
From www.slideserve.com
PPT Chapter 4 Chemical Reactions An Introduction PowerPoint Magnesium And Potassium Nitrate Reaction Magnesium is a more reactive metal than copper. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. Kno3 + mgso4 = mg(no3)2 + k2so4. Magnesium And Potassium Nitrate Reaction.
From www.chegg.com
Solved er on test tube ervations when potassium hydroxide Magnesium And Potassium Nitrate Reaction Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. We described a precipitation reaction in which a colorless solution of. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. Magnesium is a more reactive metal. Magnesium And Potassium Nitrate Reaction.
From socratic.org
What occurs when magnesium nitrate is mixed with potassium sulfate in Magnesium And Potassium Nitrate Reaction K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If precipitation is expected, write a. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it.. Magnesium And Potassium Nitrate Reaction.
From www.youtube.com
The combustion reaction of potassium nitrate and a gummy bear YouTube Magnesium And Potassium Nitrate Reaction The potassium and nitrate ions. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. We described a precipitation reaction in which a colorless solution of. Magnesium is a more reactive metal than copper. Predict the result. Magnesium And Potassium Nitrate Reaction.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Magnesium And Potassium Nitrate Reaction We described a precipitation reaction in which a colorless solution of. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. The potassium and nitrate ions. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. A precipitation reaction is a. Magnesium And Potassium Nitrate Reaction.
From www.slideserve.com
PPT Thermal PowerPoint Presentation ID544610 Magnesium And Potassium Nitrate Reaction When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. The potassium and nitrate ions. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. We described a. Magnesium And Potassium Nitrate Reaction.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Magnesium And Potassium Nitrate Reaction Magnesium is a more reactive metal than copper. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The potassium and nitrate ions. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. When a strip of magnesium metal is placed in an aqueous solution of. Magnesium And Potassium Nitrate Reaction.
From www.coursehero.com
[Solved] Does magnesium nitrate and sulfuric acid react together? If so Magnesium And Potassium Nitrate Reaction Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. We described a precipitation reaction in which a colorless solution of. Magnesium is a more reactive metal than copper. If precipitation is expected, write a. The potassium and nitrate ions. A precipitation. Magnesium And Potassium Nitrate Reaction.
From www.youtube.com
Oxidation of sugar with potassium nitrate YouTube Magnesium And Potassium Nitrate Reaction K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. We described a precipitation. Magnesium And Potassium Nitrate Reaction.
From www.researchgate.net
Scheme 3. Nitration of phenols by potassium nitrate or sodium nitrite Magnesium And Potassium Nitrate Reaction Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If precipitation is expected, write a. We described a precipitation reaction in which a colorless solution of. K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. Magnesium is a more reactive metal. Magnesium And Potassium Nitrate Reaction.
From www.numerade.com
SOLVED 'er on test tube hydroxide and magnesium nitrate were mixed Magnesium And Potassium Nitrate Reaction Magnesium is a more reactive metal than copper. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh. Magnesium And Potassium Nitrate Reaction.
From www.slideserve.com
PPT CHEMICAL REACTIONS PowerPoint Presentation, free download ID Magnesium And Potassium Nitrate Reaction When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. We described a precipitation reaction in which a. Magnesium And Potassium Nitrate Reaction.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Magnesium And Potassium Nitrate Reaction Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. If precipitation is expected, write a. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. We described a precipitation reaction in which a colorless solution of. K +. Magnesium And Potassium Nitrate Reaction.
From signalticket9.pythonanywhere.com
Glory What Happens When Magnesium Reacts With Steam Formula Of Time Period Magnesium And Potassium Nitrate Reaction A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. Magnesium is a more reactive metal than copper. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two. Magnesium And Potassium Nitrate Reaction.
From www.nagwa.com
Question Video Writing the Symbol and the Ionic Equations for a Magnesium And Potassium Nitrate Reaction Magnesium is a more reactive metal than copper. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. We described a precipitation reaction in which a colorless solution of. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. A precipitation reaction. Magnesium And Potassium Nitrate Reaction.
From www.youtube.com
Potassium Nitrate Burn Test Magnesium (Mg) YouTube Magnesium And Potassium Nitrate Reaction K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. Magnesium is a more reactive metal than copper. The potassium and nitrate ions. A precipitation reaction is a reaction. Magnesium And Potassium Nitrate Reaction.
From www.coursehero.com
[Solved] Magnesium nitrate reacts with barium in a single displacement Magnesium And Potassium Nitrate Reaction Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If precipitation is expected, write a. K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. We described a precipitation reaction in which a colorless solution of. Magnesium nitrate + potassium hydroxide =. Magnesium And Potassium Nitrate Reaction.
From www.slideshare.net
Action of heat on chemical compound e book Magnesium And Potassium Nitrate Reaction Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Magnesium is a more reactive metal than copper. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two. Magnesium And Potassium Nitrate Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for Mg(NO3)2 + KOH = KNO3 + Mg(OH)2 Magnesium And Potassium Nitrate Reaction We described a precipitation reaction in which a colorless solution of. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. If precipitation is expected, write a. Predict the result of. Magnesium And Potassium Nitrate Reaction.
From www.numerade.com
SOLVED Complete the following instructions for the problems below I Magnesium And Potassium Nitrate Reaction Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. If precipitation is expected, write a. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. Magnesium nitrate + potassium hydroxide = magnesium hydroxide. Magnesium And Potassium Nitrate Reaction.
From www.slideserve.com
PPT Double Replacement Reactions PowerPoint Presentation, free Magnesium And Potassium Nitrate Reaction Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. Magnesium is a more reactive metal than copper. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. We described a precipitation reaction in which a colorless solution of.. Magnesium And Potassium Nitrate Reaction.
From www.youtube.com
Demonstration Precipitation Reaction of Potassium Iodide and Lead Magnesium And Potassium Nitrate Reaction We described a precipitation reaction in which a colorless solution of. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. Magnesium is a more reactive metal than copper. When a strip of. Magnesium And Potassium Nitrate Reaction.
From www.numerade.com
SOLVED Write the complete balanced equation, total ionic equation and Magnesium And Potassium Nitrate Reaction K + mg(no3)2 = kno3 + mg is a single displacement (substitution) reaction where two moles of solid potassium [k] and one mole of. Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. If precipitation is expected, write a. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. The potassium. Magnesium And Potassium Nitrate Reaction.
From www.numerade.com
SOLVEDConsider the reaction between 0.156 L of 0.105 M magnesium Magnesium And Potassium Nitrate Reaction Magnesium is a more reactive metal than copper. When a strip of magnesium metal is placed in an aqueous solution of copper (ii) nitrate, it. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions. Magnesium And Potassium Nitrate Reaction.
From www.youtube.com
Potassium nitrate+sugar reaction YouTube Magnesium And Potassium Nitrate Reaction Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. Magnesium is a more reactive metal than copper. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh = mg(oh)2 + kno3 is a double. When a strip of magnesium metal is. Magnesium And Potassium Nitrate Reaction.
From eureka-patsnap-com.libproxy.mit.edu
Method for making potassium nitrate using magnesium hydroxide circular Magnesium And Potassium Nitrate Reaction Magnesium ions combine with hydroxide ions to form solid magnesium hydroxide. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium nitrate [kno 3]. Magnesium nitrate + potassium hydroxide = magnesium hydroxide + potassium nitrate mg(no3)2 + koh. Magnesium And Potassium Nitrate Reaction.