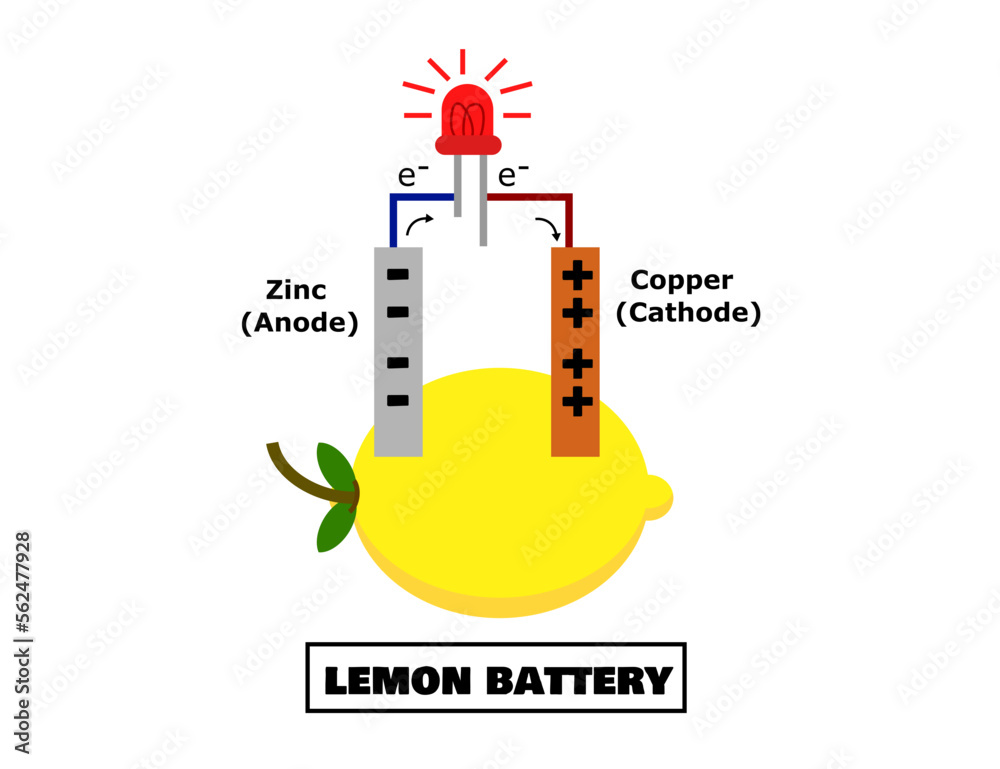
Why Are Zinc And Copper Used In Batteries . A coordinating hydrogel is used as electrolyte. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge.
from stock.adobe.com
Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. A coordinating hydrogel is used as electrolyte. A cell using zinc from a galvanised nail and a copper wire produces about 1 v.
Lemon battery.Zinc and copper.Anode and cathode.Free energy electricity
Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A coordinating hydrogel is used as electrolyte. A cell using zinc from a galvanised nail and a copper wire produces about 1 v.
From www.sliderbase.com
Electrochemical Terminology Why Are Zinc And Copper Used In Batteries A cell using zinc from a galvanised nail and a copper wire produces about 1 v. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A coordinating hydrogel is used. Why Are Zinc And Copper Used In Batteries.
From zincfive.com
5 Benefits of ZincFive NickelZinc Batteries ZincFive Why Are Zinc And Copper Used In Batteries A coordinating hydrogel is used as electrolyte. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed. Why Are Zinc And Copper Used In Batteries.
From spark.adobe.com
Zinc and Copper Chloride Reaction Why Are Zinc And Copper Used In Batteries A cell using zinc from a galvanised nail and a copper wire produces about 1 v. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use. Why Are Zinc And Copper Used In Batteries.
From stock.adobe.com
Lemon battery.Zinc and copper.Anode and cathode.Free energy electricity Why Are Zinc And Copper Used In Batteries If you make three cells like this, using vinegar as the acid, and join them up in series to form a. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge.. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. A coordinating hydrogel is used. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
Separating Copper and Zinc Alloys A StepbyStep Guide Why Are Zinc And Copper Used In Batteries Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. A coordinating hydrogel is used as electrolyte. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. When zinc foil was used as an. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
CopperZinc Alloys An Overview Why Are Zinc And Copper Used In Batteries If you make three cells like this, using vinegar as the acid, and join them up in series to form a. A coordinating hydrogel is used as electrolyte. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A cell using zinc from a galvanised nail and a copper. Why Are Zinc And Copper Used In Batteries.
From pixels.com
Zinccopper Battery Photograph by Andrew Lambert Photography Why Are Zinc And Copper Used In Batteries A cell using zinc from a galvanised nail and a copper wire produces about 1 v. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. Since the first battery was. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. If you make three cells. Why Are Zinc And Copper Used In Batteries.
From www.advancedsciencenews.com
A New Solution to an Old Challenge Recharging CuZn Batteries Why Are Zinc And Copper Used In Batteries When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A coordinating hydrogel is used as electrolyte. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. Since the first battery was invented in 1799 using only copper and zinc, researchers. Why Are Zinc And Copper Used In Batteries.
From stock.adobe.com
Vector illustration of the galvanic cell element. Battery diagram with Why Are Zinc And Copper Used In Batteries If you make three cells like this, using vinegar as the acid, and join them up in series to form a. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. Since the. Why Are Zinc And Copper Used In Batteries.
From www.change-climate.com
BATTERIES BATTERY STORAGE FORMATS Why Are Zinc And Copper Used In Batteries Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. If you make three cells like this, using vinegar as the acid, and join. Why Are Zinc And Copper Used In Batteries.
From www.mdpi.com
Batteries Free FullText Secondary ZincAir Batteries A View on Why Are Zinc And Copper Used In Batteries When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. A coordinating hydrogel is used as electrolyte. Since the first battery was invented in 1799 using only copper. Why Are Zinc And Copper Used In Batteries.
From my.cytron.io
Copper & Zinc Plate Fruit Battery Experiment Kit Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use. Why Are Zinc And Copper Used In Batteries.
From saylordotorg.github.io
Describing Electrochemical Cells Why Are Zinc And Copper Used In Batteries A cell using zinc from a galvanised nail and a copper wire produces about 1 v. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. When zinc foil was used. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
Zinc vs. Copper What's the Difference Why Are Zinc And Copper Used In Batteries A coordinating hydrogel is used as electrolyte. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed. Why Are Zinc And Copper Used In Batteries.
From www.researchgate.net
Hector PEREZ Doctor of Philosophy Engineering University of Why Are Zinc And Copper Used In Batteries A cell using zinc from a galvanised nail and a copper wire produces about 1 v. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. When zinc foil was used as an electrode, over 80 percent of the zinc ended up. Why Are Zinc And Copper Used In Batteries.
From www.instructables.com
Wire Coil Zinc Copper Battery 4 Steps (with Pictures) Instructables Why Are Zinc And Copper Used In Batteries A coordinating hydrogel is used as electrolyte. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. If you make three cells like this, using vinegar as the acid, and join them up in series to. Why Are Zinc And Copper Used In Batteries.
From ar.inspiredpencil.com
Zinc Battery Why Are Zinc And Copper Used In Batteries Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. Rechargeable aqueous zinc metal batteries represent a promising solution to the. Why Are Zinc And Copper Used In Batteries.
From www.youtube.com
Copper, Zinc and Guinness Battery YouTube Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. A coordinating hydrogel is used as electrolyte. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. Since the first battery was invented in 1799 using only copper and zinc, researchers. Why Are Zinc And Copper Used In Batteries.
From www.youtube.com
Nickelzinc battery YouTube Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. A coordinating hydrogel is used. Why Are Zinc And Copper Used In Batteries.
From en.ppt-online.org
Primary Batteries. The zinccarbon cell online presentation Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. A coordinating hydrogel is used as electrolyte. If you make three cells like this,. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
Difference Between Copper and Zinc Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. A cell using zinc from a galvanised nail and a copper wire produces about. Why Are Zinc And Copper Used In Batteries.
From www.alamy.com
Principle of a rechargeable copperzinc battery. Used in chemistry Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. A coordinating hydrogel is used as electrolyte. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. When zinc foil was used as an electrode, over 80 percent of the zinc. Why Are Zinc And Copper Used In Batteries.
From colouremployer8.gitlab.io
Marvelous Copper Zinc Battery Reaction Cie Chemistry A Level Syllabus Why Are Zinc And Copper Used In Batteries Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. A coordinating hydrogel is used as electrolyte. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A cell using zinc from a galvanised nail and a copper wire produces about. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
CopperZinc Alloys A Complete Guide Why Are Zinc And Copper Used In Batteries If you make three cells like this, using vinegar as the acid, and join them up in series to form a. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique. Why Are Zinc And Copper Used In Batteries.
From www.takomabattery.com
Zinc manganese battery types and application The Best lithium ion Why Are Zinc And Copper Used In Batteries Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A cell using zinc from a galvanised nail and a copper. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
Comparing the Reactivity of Copper vs Zinc Why Are Zinc And Copper Used In Batteries A cell using zinc from a galvanised nail and a copper wire produces about 1 v. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. When zinc foil was used. Why Are Zinc And Copper Used In Batteries.
From www.slideserve.com
PPT OxidationReduction Reactions PowerPoint Presentation, free Why Are Zinc And Copper Used In Batteries Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. A cell using zinc from a galvanised nail and a copper. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
A Breakdown of Copper vs. Zinc Reactivity Why Are Zinc And Copper Used In Batteries When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. A coordinating hydrogel is used as electrolyte. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed. Why Are Zinc And Copper Used In Batteries.
From www.dreamstime.com
ZincSilver Battery Zincsilver Batteries are a Type of Primary Battery Why Are Zinc And Copper Used In Batteries A cell using zinc from a galvanised nail and a copper wire produces about 1 v. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. Since the first battery was. Why Are Zinc And Copper Used In Batteries.
From www.youtube.com
Zinc Air Battery Working Advantages of Zinc Air Batteries YouTube Why Are Zinc And Copper Used In Batteries A coordinating hydrogel is used as electrolyte. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on the gigawatt. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. When zinc foil was used as an. Why Are Zinc And Copper Used In Batteries.
From blog.thepipingmart.com
Copper Zinc Alloys Properties and Uses Why Are Zinc And Copper Used In Batteries A cell using zinc from a galvanised nail and a copper wire produces about 1 v. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique. Why Are Zinc And Copper Used In Batteries.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry for Majors Why Are Zinc And Copper Used In Batteries Since the first battery was invented in 1799 using only copper and zinc, researchers have harnessed many other elements, each with its unique properties, for use in batteries. If you make three cells like this, using vinegar as the acid, and join them up in series to form a. A coordinating hydrogel is used as electrolyte. When zinc foil was. Why Are Zinc And Copper Used In Batteries.
From www.electricity-magnetism.org
Zinccarbon Battery Leclanché cell Electricity Why Are Zinc And Copper Used In Batteries A coordinating hydrogel is used as electrolyte. A cell using zinc from a galvanised nail and a copper wire produces about 1 v. When zinc foil was used as an electrode, over 80 percent of the zinc ended up being used for the discharge. Rechargeable aqueous zinc metal batteries represent a promising solution to the storage of renewable energy on. Why Are Zinc And Copper Used In Batteries.