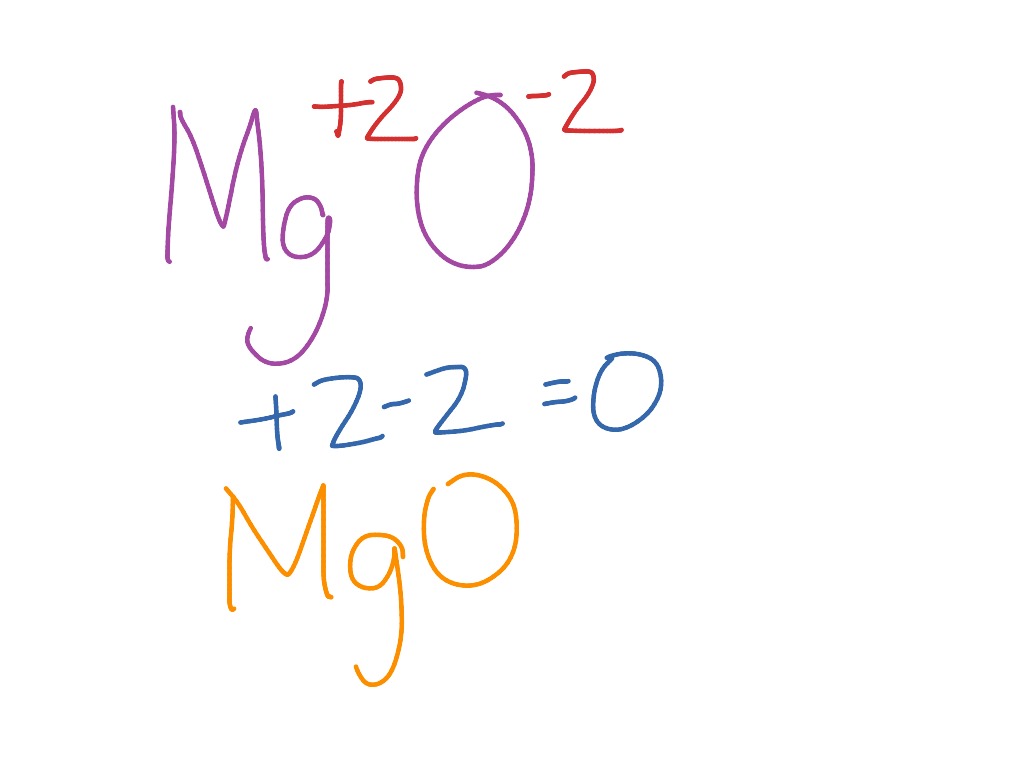Magnesium Oxide + Water Formula . Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Magnesium hydroxide → magnesium oxide +. To synthesize a compound containing magnesium and oxygen, and to. Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; Mass of magnesium + mass of oxygen = mass of. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. In the case of the combustion of magnesium, the following equation must be true: Determining the empirical formula of magnesium oxide.
from www.showme.com
Magnesium hydroxide → magnesium oxide +. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Mass of magnesium + mass of oxygen = mass of. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. To synthesize a compound containing magnesium and oxygen, and to. Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; In the case of the combustion of magnesium, the following equation must be true: Determining the empirical formula of magnesium oxide. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or.
Formula for Magnesium oxide Science, Chemistry ShowMe
Magnesium Oxide + Water Formula Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Determining the empirical formula of magnesium oxide. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. To synthesize a compound containing magnesium and oxygen, and to. In the case of the combustion of magnesium, the following equation must be true: Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; Magnesium hydroxide → magnesium oxide +. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Mass of magnesium + mass of oxygen = mass of.
From www.numerade.com
SOLVED Solid magnesium hydroxide into gaseous water and Magnesium Oxide + Water Formula Magnesium hydroxide → magnesium oxide +. Mass of magnesium + mass of oxygen = mass of. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Determining. Magnesium Oxide + Water Formula.
From exopptltv.blob.core.windows.net
Magnesium Hydroxide Ionic Or Covalent at Gene Garst blog Magnesium Oxide + Water Formula Determining the empirical formula of magnesium oxide. In the case of the combustion of magnesium, the following equation must be true: Magnesium hydroxide → magnesium oxide +. To synthesize a compound containing magnesium and oxygen, and to. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and.. Magnesium Oxide + Water Formula.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium Oxide + Water Formula Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. In the case of the combustion of magnesium, the following equation must be true: Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. The empirical formula of a compound merely gives the ratio of atoms in the compound based. Magnesium Oxide + Water Formula.
From www.researchgate.net
Thermal analysis of magnesium oxide and water glass mixture. Download Magnesium Oxide + Water Formula Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Determining the empirical formula of magnesium oxide. In the case of the combustion of magnesium, the following equation must be true: To synthesize a compound containing magnesium and oxygen, and to. The empirical formula of a compound. Magnesium Oxide + Water Formula.
From www.numerade.com
SOLVED Write a balanced equation for reaction of the basic oxide Magnesium Oxide + Water Formula Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium hydroxide → magnesium oxide +. To synthesize a. Magnesium Oxide + Water Formula.
From www.nagwa.com
Question Video Determining the Color of Litmus Solution When Added to Magnesium Oxide + Water Formula To synthesize a compound containing magnesium and oxygen, and to. Determining the empirical formula of magnesium oxide. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Mass of magnesium + mass of oxygen = mass of.. Magnesium Oxide + Water Formula.
From www.youtube.com
Empirical Formula of Magnesium Oxide Postlab Analysis YouTube Magnesium Oxide + Water Formula Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. To synthesize a compound containing magnesium and oxygen, and to. In the case of the combustion of magnesium, the following equation must be true: Magnesium hydroxide → magnesium oxide +. Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula. Magnesium Oxide + Water Formula.
From www.youtube.com
How to Balance MgO + H2O = Mg(OH)2 (Magnesium Oxide plus Water) YouTube Magnesium Oxide + Water Formula Determining the empirical formula of magnesium oxide. To synthesize a compound containing magnesium and oxygen, and to. Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; Magnesium hydroxide → magnesium oxide +. Magnesium oxide, also known as magnesia, is a classic. Magnesium Oxide + Water Formula.
From childhealthpolicy.vumc.org
Chemical equation for magnesium oxide. Magnesium oxide lab. 20221010 Magnesium Oxide + Water Formula In the case of the combustion of magnesium, the following equation must be true: To synthesize a compound containing magnesium and oxygen, and to. Determining the empirical formula of magnesium oxide. Mass of magnesium + mass of oxygen = mass of. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium. Magnesium Oxide + Water Formula.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Reaction Between Magnesium And Oxygen Gas Magnesium Oxide + Water Formula Magnesium hydroxide → magnesium oxide +. Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; Determining the empirical formula of magnesium oxide. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Magnesium oxide, also known as magnesia, is a classic. Magnesium Oxide + Water Formula.
From infinitylearn.com
Magnesium hydroxide Formula Infinity Learn Magnesium Oxide + Water Formula In the case of the combustion of magnesium, the following equation must be true: Magnesium hydroxide → magnesium oxide +. To synthesize a compound containing magnesium and oxygen, and to. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. Magnesium oxide, also known as. Magnesium Oxide + Water Formula.
From studylib.net
Chem 132.L7. Simplest Formula of Magnesium Oxide Magnesium Oxide + Water Formula The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. Magnesium hydroxide → magnesium oxide +. Determining the empirical formula of magnesium oxide. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Magnesium nitride. Magnesium Oxide + Water Formula.
From olympiapublishers.com
Empirical Formula Of The Oxide Of Magnesium Magnesium Oxide + Water Formula Determining the empirical formula of magnesium oxide. Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or.. Magnesium Oxide + Water Formula.
From studylib.net
Determining the Empirical Formula of Magnesium Oxide Magnesium Oxide + Water Formula Magnesium hydroxide → magnesium oxide +. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. To synthesize a compound containing magnesium and oxygen, and to. Determining the empirical formula of magnesium oxide. Magnesium oxide. Magnesium Oxide + Water Formula.
From ar.inspiredpencil.com
Magnesium And Water Reaction Magnesium Oxide + Water Formula Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. To synthesize a compound containing magnesium and oxygen, and to. Mass of magnesium + mass of oxygen = mass of. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such. Magnesium Oxide + Water Formula.
From www.youtube.com
How to Write the Formula for MgO (Magnesium oxide) YouTube Magnesium Oxide + Water Formula Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. Magnesium oxide, also known as magnesia, is. Magnesium Oxide + Water Formula.
From www.youtube.com
Mg(OH)2+HNO3=Mg(NO3)2+H2O Balanced EquationMagnesium Hydroxide+Nitric Magnesium Oxide + Water Formula The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Mass of magnesium + mass of oxygen = mass of. Magnesium hydroxide → magnesium oxide. Magnesium Oxide + Water Formula.
From www.youtube.com
Reaction of Magnesium and Water (Mg + H2O) YouTube Magnesium Oxide + Water Formula Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Determining the empirical formula of magnesium oxide. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. In the case of the combustion of magnesium, the following equation must be true: Magnesium oxide, also known as magnesia, is a classic. Magnesium Oxide + Water Formula.
From www.youtube.com
How to write chemical formula of Magnesium oxide? YouTube Magnesium Oxide + Water Formula Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. In the case of the combustion of magnesium, the following equation must be true: Determining the empirical formula of magnesium oxide. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Magnesium oxide has the chemical formula. Magnesium Oxide + Water Formula.
From www.slideserve.com
PPT Mg(OH) 2 + 2HCl → 2H 2 O + MgCl 2 PowerPoint Presentation, free Magnesium Oxide + Water Formula Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; To synthesize a compound containing magnesium and oxygen, and to. Determining the empirical formula of magnesium oxide. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which. Magnesium Oxide + Water Formula.
From www.youtube.com
Synthesis of Magnesium Oxide YouTube Magnesium Oxide + Water Formula In the case of the combustion of magnesium, the following equation must be true: Mass of magnesium + mass of oxygen = mass of. Determining the empirical formula of magnesium oxide. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Magnesium oxide has the chemical formula mgo, and contains. Magnesium Oxide + Water Formula.
From www.toppr.com
Magnesium Oxide Formula Definition, Concepts and Examples Magnesium Oxide + Water Formula Mass of magnesium + mass of oxygen = mass of. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. To synthesize a compound containing magnesium and oxygen, and to. The empirical formula of a compound merely. Magnesium Oxide + Water Formula.
From brainly.in
What happens when Magnesium oxide is dissolved in water? Write a word Magnesium Oxide + Water Formula Mass of magnesium + mass of oxygen = mass of. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium hydroxide → magnesium oxide +. Determining the empirical formula of magnesium oxide. Magnesium oxide, often simply. Magnesium Oxide + Water Formula.
From www.chegg.com
Solved Write a balanced formula equation for magnesium Magnesium Oxide + Water Formula The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg). Magnesium Oxide + Water Formula.
From www.sciencephoto.com
Magnesium oxide reacts with water Stock Image C030/8188 Science Magnesium Oxide + Water Formula In the case of the combustion of magnesium, the following equation must be true: Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Determining the empirical formula of magnesium oxide. The empirical formula of a compound merely gives the ratio of atoms in the compound based. Magnesium Oxide + Water Formula.
From www.shutterstock.com
Magnesium Oxide Formula Mgo Compound Stock Illustration Magnesium Oxide + Water Formula Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+). Magnesium Oxide + Water Formula.
From www.youtube.com
Net Ionic Equation for Mg + HCl (Magnesium + Hydrochloric acid) YouTube Magnesium Oxide + Water Formula Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. In the case of the combustion of magnesium, the following equation must be true: To synthesize a compound containing magnesium and oxygen, and to. The empirical formula. Magnesium Oxide + Water Formula.
From www.tes.com
Empirical Formula of Magnesium Oxide GCSE Lesson (SC9a CC9a) Teaching Magnesium Oxide + Water Formula Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. Mass of magnesium + mass of oxygen = mass of. To synthesize a compound containing magnesium and oxygen, and to. Magnesium oxide, often simply referred. Magnesium Oxide + Water Formula.
From www.slideshare.net
Reactions & Formulas Magnesium Oxide + Water Formula Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; Mass of magnesium + mass of oxygen = mass of. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. To synthesize a compound containing magnesium and oxygen, and to. Determining the. Magnesium Oxide + Water Formula.
From cartoondealer.com
Magnesium Oxide Chemical Formula On Waterdrop Background Stock Image Magnesium Oxide + Water Formula The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. In the case of the combustion of magnesium, the following equation must be true: Magnesium. Magnesium Oxide + Water Formula.
From healthjade.com
Magnesium Oxide Uses, Benefits, Dosage, Side Effects Magnesium Oxide + Water Formula Determining the empirical formula of magnesium oxide. In the case of the combustion of magnesium, the following equation must be true: Mass of magnesium + mass of oxygen = mass of. To synthesize a compound containing magnesium and oxygen, and to. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium. Magnesium Oxide + Water Formula.
From mungfali.com
PPT Section 9.1 Naming Ions PowerPoint Presentation Magnesium Oxide + Water Formula Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Magnesium oxide has the chemical formula mgo, and contains only magnesium (mg) and oxygen (o) sodium oxide has the formula na₂o and contains only sodium (na) and oxygen; Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. In the. Magnesium Oxide + Water Formula.
From www.showme.com
Formula for Magnesium oxide Science, Chemistry ShowMe Magnesium Oxide + Water Formula Mass of magnesium + mass of oxygen = mass of. To synthesize a compound containing magnesium and oxygen, and to. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations. Magnesium Oxide + Water Formula.
From www.tessshebaylo.com
The Chemical Equation For Synthesis Of Magnesium Oxide From And Oxygen Magnesium Oxide + Water Formula To synthesize a compound containing magnesium and oxygen, and to. Magnesium oxide, also known as magnesia, is a classic example of an ionic compound, which is composed of magnesium cations (mg 2+) and. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role. Magnesium Oxide + Water Formula.
From www.numerade.com
SOLVEDMagnesium hydroxide is formed from the reaction of magnesium Magnesium Oxide + Water Formula To synthesize a compound containing magnesium and oxygen, and to. Magnesium nitride + water → magnesium hydroxide + ammonia (rxn. Magnesium oxide, often simply referred to as ‘magnesia’, is an inorganic compound that has a significant role in various industrial,. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such. Magnesium Oxide + Water Formula.