Why Does Calcium Carbide React With Water . Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Quite a bit of energy is stored in that acetylene molecule,. So there is no lewis reaction involving the calcium. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Cac 2 + [s] fe → cas + 2 [c] fe. Calcium cyanamide can also be produced from this. Calcium, for example, reacts fairly vigorously and exothermically with cold water. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. This reaction is used to prepare.
from www.slideserve.com
Quite a bit of energy is stored in that acetylene molecule,. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium cyanamide can also be produced from this. So there is no lewis reaction involving the calcium. Cac 2 + [s] fe → cas + 2 [c] fe. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. This reaction is used to prepare.
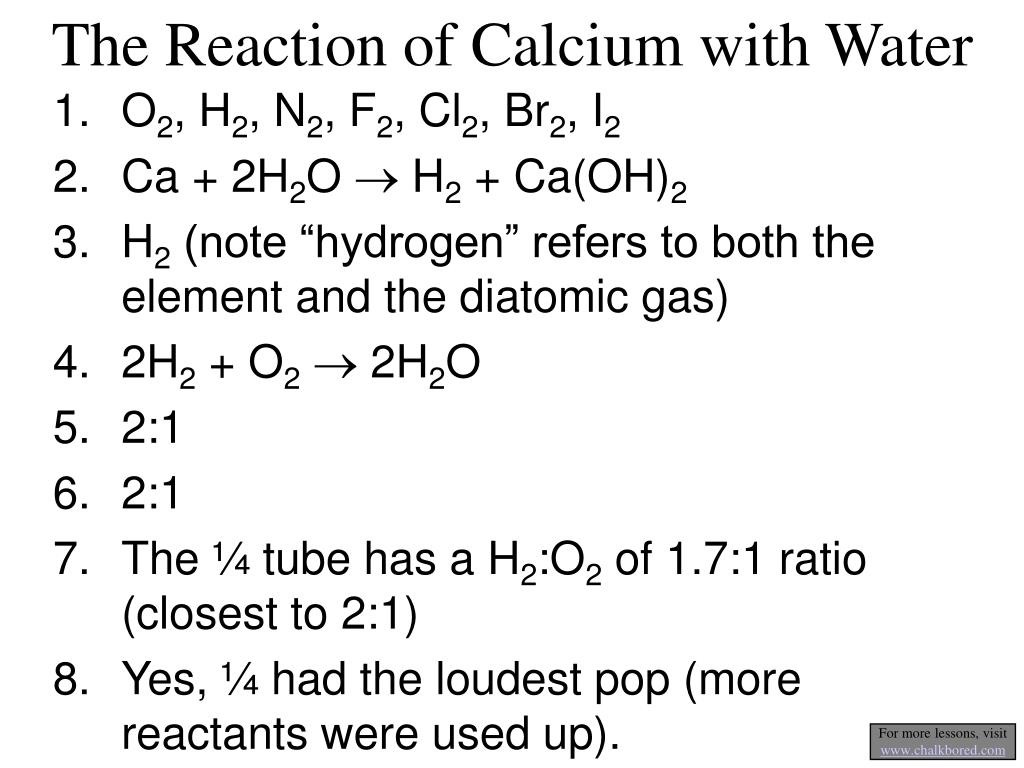
PPT The Reaction of Calcium with Water PowerPoint Presentation, free
Why Does Calcium Carbide React With Water Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Quite a bit of energy is stored in that acetylene molecule,. Calcium, for example, reacts fairly vigorously and exothermically with cold water. This reaction is used to prepare. Cac 2 + [s] fe → cas + 2 [c] fe. Calcium cyanamide can also be produced from this. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. So there is no lewis reaction involving the calcium.
From www.coursehero.com
[Solved] Calcium carbide (CaC 2) reacts with water to produce acetylene Why Does Calcium Carbide React With Water Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: This reaction is used to prepare. Calcium carbide (cac2). Why Does Calcium Carbide React With Water.
From www.youtube.com
Calcium carbide and Water Reaction YouTube Why Does Calcium Carbide React With Water Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: So there is no lewis reaction involving the calcium. Quite a bit of energy is stored in that acetylene molecule,. Calcium, for example, reacts fairly vigorously and exothermically with cold water. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide). Why Does Calcium Carbide React With Water.
From www.tessshebaylo.com
Balanced Equation For The Reaction Of Calcium Carbide With Water Why Does Calcium Carbide React With Water Calcium cyanamide can also be produced from this. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and. Why Does Calcium Carbide React With Water.
From www.youtube.com
calcium carbide and water experiment Kuch dekhte hain dekhte hain Why Does Calcium Carbide React With Water Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: This reaction is used to prepare. Calcium, for example, reacts fairly vigorously and exothermically with cold water. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Cac 2 + [s] fe → cas + 2 [c] fe. Calcium carbide is not. Why Does Calcium Carbide React With Water.
From www.youtube.com
Experiment With Calcium Carbide and Water _ Diy Science Experiment Why Does Calcium Carbide React With Water Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Calcium cyanamide can also be produced from this. Quite a bit of energy is stored in that acetylene molecule,. Cac 2 + [s] fe → cas + 2 [c] fe. This reaction is used to prepare. Calcium. Why Does Calcium Carbide React With Water.
From www.chegg.com
Consider the reaction of Calcium carbide and water as Why Does Calcium Carbide React With Water Calcium, for example, reacts fairly vigorously and exothermically with cold water. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Cac 2 + [s] fe → cas + 2 [c] fe. Quite a bit of energy is stored in that acetylene molecule,. This reaction is used to prepare. Calcium carbide reacts with sulfur in. Why Does Calcium Carbide React With Water.
From www.youtube.com
Calcium Carbide and Water & Combustion of Acetylene YouTube Why Does Calcium Carbide React With Water Quite a bit of energy is stored in that acetylene molecule,. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: So there is no lewis reaction involving the calcium. Calcium carbide can be reacted with water to produce. Why Does Calcium Carbide React With Water.
From solvedlib.com
Calcium carbide (CaC2) reacts with water to produce a… SolvedLib Why Does Calcium Carbide React With Water Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium, for example, reacts fairly vigorously and exothermically with cold water. Quite a bit of energy is stored in that acetylene molecule,. So there is no lewis reaction involving the calcium.. Why Does Calcium Carbide React With Water.
From carbidecalcium.com
calcium carbide and water chemical equation calcium carbide appearance Why Does Calcium Carbide React With Water Quite a bit of energy is stored in that acetylene molecule,. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium cyanamide can also be produced from this. So there is no lewis reaction involving the calcium. Calcium, for example, reacts fairly vigorously and exothermically with cold water. Cac 2 + [s] fe → cas +. Why Does Calcium Carbide React With Water.
From melscience.com
Chemical characteristics of calcium carbide and its reaction with water Why Does Calcium Carbide React With Water Calcium, for example, reacts fairly vigorously and exothermically with cold water. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Calcium carbide reacts with sulfur. Why Does Calcium Carbide React With Water.
From www.youtube.com
How to Balance CaC2 + H2O = Ca(OH)2 + C2H2 (Calcium carbide + Water Why Does Calcium Carbide React With Water Calcium cyanamide can also be produced from this. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Cac 2 + [s] fe → cas + 2 [c] fe. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Calcium carbide (cac2) hydrolysis and. Why Does Calcium Carbide React With Water.
From www.youtube.com
Calcium Carbide and Water Experiment YouTube Why Does Calcium Carbide React With Water Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. So there is no lewis reaction involving the calcium. Calcium cyanamide can also be produced from this. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. This reaction is used to prepare. Cac 2 + [s] fe → cas +. Why Does Calcium Carbide React With Water.
From www.youtube.com
What Are Exothermic Reactions Chemistry Calcium carbide in water Why Does Calcium Carbide React With Water So there is no lewis reaction involving the calcium. Calcium, for example, reacts fairly vigorously and exothermically with cold water. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. This reaction is used to prepare. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield. Why Does Calcium Carbide React With Water.
From solvedlib.com
10. Calcium carbide (CaC2) reacts unbalanced reaction… SolvedLib Why Does Calcium Carbide React With Water Calcium, for example, reacts fairly vigorously and exothermically with cold water. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium carbide is not volatile. Why Does Calcium Carbide React With Water.
From www.tessshebaylo.com
Balanced Equation For The Reaction Of Calcium Carbide With Water Why Does Calcium Carbide React With Water Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Quite a bit of energy is stored in that acetylene molecule,. This reaction is. Why Does Calcium Carbide React With Water.
From www.youtube.com
Calcium carbide and water reaction Calcium carbide and water YouTube Why Does Calcium Carbide React With Water Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Calcium cyanamide can also be produced from this. This reaction is used to prepare. Cac 2 + [s] fe → cas +. Why Does Calcium Carbide React With Water.
From www.youtube.com
Class11Cac2+H2O=acetylene (calcium carbide react with Why Does Calcium Carbide React With Water Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide (cac2) hydrolysis and react in the. Why Does Calcium Carbide React With Water.
From carbidecalcium.com
calcium carbide and water chemical equation calcium carbide appearance Why Does Calcium Carbide React With Water Calcium, for example, reacts fairly vigorously and exothermically with cold water. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Cac 2 + [s] fe → cas + 2 [c] fe. This reaction is used to prepare. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water. Why Does Calcium Carbide React With Water.
From www.coursehero.com
[Solved] Calcium carbide reacts with water to produce acetylene gas Why Does Calcium Carbide React With Water Calcium, for example, reacts fairly vigorously and exothermically with cold water. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. So there is no lewis reaction involving the calcium. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide reacts with sulfur in molten iron to. Why Does Calcium Carbide React With Water.
From www.slideserve.com
PPT The Reaction of Calcium with Water PowerPoint Presentation, free Why Does Calcium Carbide React With Water Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Quite a bit of energy is stored in that acetylene molecule,. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2),. Why Does Calcium Carbide React With Water.
From www.youtube.com
gas stoichiometry calcium carbide and water YouTube Why Does Calcium Carbide React With Water Calcium, for example, reacts fairly vigorously and exothermically with cold water. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene. Why Does Calcium Carbide React With Water.
From www.youtube.com
Calcium Carbide Reaction with Water YouTube Why Does Calcium Carbide React With Water Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium, for example, reacts fairly vigorously and exothermically with cold water. This reaction is used to prepare. Calcium cyanamide can also be produced from this. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas. Why Does Calcium Carbide React With Water.
From www.youtube.com
Calcium carbide reacts with water to produce YouTube Why Does Calcium Carbide React With Water Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. This reaction is used to prepare. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Cac 2 + [s] fe → cas +. Why Does Calcium Carbide React With Water.
From www.youtube.com
The Reaction of Calcium Metal and Water YouTube Why Does Calcium Carbide React With Water Quite a bit of energy is stored in that acetylene molecule,. This reaction is used to prepare. Calcium cyanamide can also be produced from this. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: So there is no lewis reaction involving the calcium. Calcium carbide is not volatile and not soluble in any known. Why Does Calcium Carbide React With Water.
From www.youtube.com
CALCIUM CARBIDE AND WATER REACTION Combustion Reaction of Acetylene Why Does Calcium Carbide React With Water Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Calcium carbide (cac2) hydrolysis and react in the. Why Does Calcium Carbide React With Water.
From byjus.com
ques . Calcium carbide produces acetylene on reaction with water Why Does Calcium Carbide React With Water Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Cac 2 + [s] fe → cas + 2 [c] fe. This reaction is used to prepare. So there is no lewis reaction involving the calcium. Calcium cyanamide can also be produced from this. Calcium carbide can. Why Does Calcium Carbide React With Water.
From www.youtube.com
Dropping of water over calcium carbide produces ______gas. YouTube Why Does Calcium Carbide React With Water Cac 2 + [s] fe → cas + 2 [c] fe. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. So there is no lewis reaction involving the calcium. Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Quite a bit of energy is stored in that acetylene molecule,. This. Why Does Calcium Carbide React With Water.
From brainly.in
what happens when calcium carbide is treated with water ? give chemical Why Does Calcium Carbide React With Water Quite a bit of energy is stored in that acetylene molecule,. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Calcium cyanamide can also be produced from this. Calcium carbide can. Why Does Calcium Carbide React With Water.
From www.toppr.com
Name the product formed when calcium carbide react with water. Why Does Calcium Carbide React With Water So there is no lewis reaction involving the calcium. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and. Why Does Calcium Carbide React With Water.
From www.sciencephoto.com
Calcium Carbide reacting with Water Stock Image C002/8205 Science Why Does Calcium Carbide React With Water Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. So there is no lewis reaction involving the calcium. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium cyanamide can also be produced from this. Quite a bit of energy is stored in that acetylene molecule,. Bubbles of hydrogen gas. Why Does Calcium Carbide React With Water.
From askfilo.com
Calcium carbide produces acetylene on reaction with water. Ethylene can b.. Why Does Calcium Carbide React With Water This reaction is used to prepare. Quite a bit of energy is stored in that acetylene molecule,. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is. Why Does Calcium Carbide React With Water.
From www.transtutors.com
(Solved) 13) Calcium Carbide (CaC2) Reacts With Water To Produce Why Does Calcium Carbide React With Water This reaction is used to prepare. Cac 2 + [s] fe → cas + 2 [c] fe. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. Quite a bit of energy is stored in that acetylene molecule,. Calcium cyanamide can. Why Does Calcium Carbide React With Water.
From www.tessshebaylo.com
Balanced Chemical Equation For The Reaction Of Calcium Carbide With Why Does Calcium Carbide React With Water Calcium carbide (cac2) hydrolysis and react in the water to give acetylene (c2h2), calcium hydroxide. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Quite a bit of energy is stored in that acetylene molecule,. Cac 2 + [s] fe → cas + 2 [c] fe. So there is no lewis reaction involving the calcium. Calcium. Why Does Calcium Carbide React With Water.
From www.tessshebaylo.com
Balanced Chemical Equation For The Reaction Between Calcium Carbide And Why Does Calcium Carbide React With Water Calcium, for example, reacts fairly vigorously and exothermically with cold water. Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. This reaction is used to prepare. Cac 2 + [s] fe → cas + 2 [c] fe. Bubbles of hydrogen gas are given off, and a white precipitate (of calcium hydroxide) is formed,. So there is. Why Does Calcium Carbide React With Water.
From www.youtube.com
Ethylene from the reaction of calcium carbide and water YouTube Why Does Calcium Carbide React With Water Calcium carbide is not volatile and not soluble in any known solvent, and reacts with water to yield acetylene gas and calcium hydroxide. Calcium carbide reacts with sulfur in molten iron to form calcium sulfide and release carbon: Calcium carbide can be reacted with water to produce acetylene and calcium hydroxide. Calcium cyanamide can also be produced from this. Quite. Why Does Calcium Carbide React With Water.