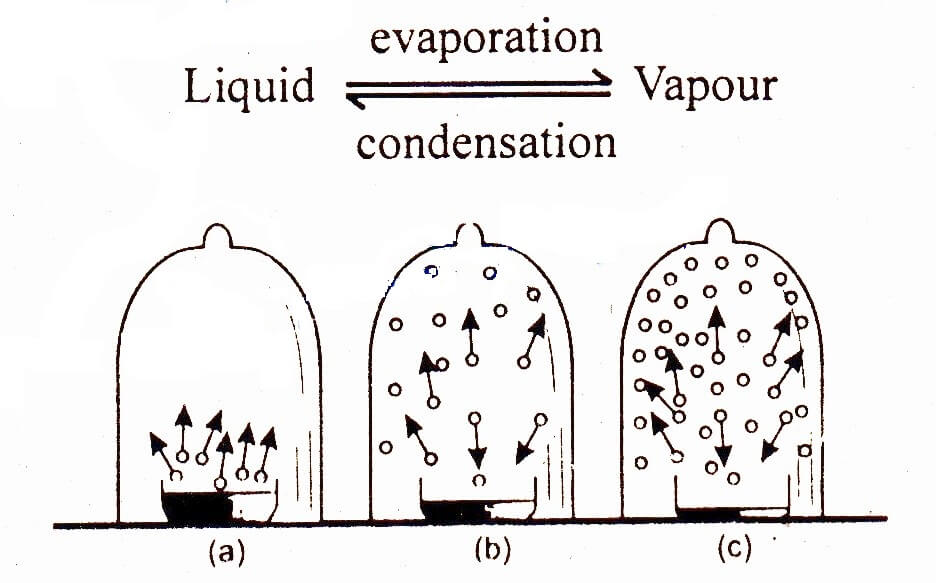
What Is Vapour Pressure Definition . The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapor pressure is a property of a liquid based on the strength of its. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container.
from chemistryskills.com
The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Vapor pressure is a property of a liquid based on the strength of its. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample.
Define and explain vapour pressure Chemistry Skills
What Is Vapour Pressure Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Vapor pressure is a property of a liquid based on the strength of its. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container.
From saylordotorg.github.io
Vapor Pressure What Is Vapour Pressure Definition Vapor pressure is a property of a liquid based on the strength of its. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed. What Is Vapour Pressure Definition.
From brainly.in
How vapour pressure lowering is related to a rise in boiling point of What Is Vapour Pressure Definition Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure is a measure of the pressure (force per unit area). What Is Vapour Pressure Definition.
From www.slideserve.com
PPT Converting Pressures PowerPoint Presentation, free download ID What Is Vapour Pressure Definition The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapor pressure is a property of a liquid based on the strength of its. Vapour pressure, pressure exerted by a vapour when the vapour. What Is Vapour Pressure Definition.
From byjus.com
What is the effect on vapour pressure of solution when external What Is Vapour Pressure Definition Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form,. What Is Vapour Pressure Definition.
From byjus.com
wht is saturated vapour pressure What Is Vapour Pressure Definition Vapor pressure is a property of a liquid based on the strength of its. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapor pressure or equilibrium vapor pressure is the pressure of. What Is Vapour Pressure Definition.
From ecoursesonline.iasri.res.in
FM LESSON 3. PROPERTIES OF FLUID What Is Vapour Pressure Definition The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is a property of a liquid based on the strength of its. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is the pressure exerted by the vapour of. What Is Vapour Pressure Definition.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Is Vapour Pressure Definition The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample.. What Is Vapour Pressure Definition.
From www.youtube.com
relative lowering of vapour pressure solutions solutions class 12 What Is Vapour Pressure Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed.. What Is Vapour Pressure Definition.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is Vapour Pressure Definition That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. What Is Vapour Pressure Definition.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Is Vapour Pressure Definition The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is a property of a liquid based on the strength of its. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapour pressure, pressure exerted. What Is Vapour Pressure Definition.
From www.youtube.com
Vapour Pressure Definition Example and Factors Explained in detail in What Is Vapour Pressure Definition Vapor pressure is a property of a liquid based on the strength of its. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed. What Is Vapour Pressure Definition.
From www.youtube.com
Vapour Pressure & Raoults Law Chemistry Class 12 IIT JEE Main What Is Vapour Pressure Definition That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a property of a liquid based on the strength of its. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Vapour pressure is the. What Is Vapour Pressure Definition.
From www.slideshare.net
Vapor pressure and boiling What Is Vapour Pressure Definition Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor. What Is Vapour Pressure Definition.
From brainly.in
what is vapour pressure? Brainly.in What Is Vapour Pressure Definition That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapour pressure, pressure exerted by a. What Is Vapour Pressure Definition.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Is Vapour Pressure Definition The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Vapor pressure is a property of a liquid based on the strength. What Is Vapour Pressure Definition.
From byjus.com
18. What is the relation between osmotic pressure (π) and relative What Is Vapour Pressure Definition Vapor pressure is a property of a liquid based on the strength of its. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. That is, the pressure of. What Is Vapour Pressure Definition.
From peacecommission.kdsg.gov.ng
Vapor Pressure Definition And How To Calculate It What Is Vapour Pressure Definition Vapor pressure is a property of a liquid based on the strength of its. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a. What Is Vapour Pressure Definition.
From www.youtube.com
Vapour Pressure And Cavitation Basic Concepts Fluid Properties What Is Vapour Pressure Definition Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form,. What Is Vapour Pressure Definition.
From www.youtube.com
MECHANICAL ENGINEERING WHAT IS VAPOUR PRESSURE? YouTube What Is Vapour Pressure Definition Vapor pressure is a property of a liquid based on the strength of its. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure is the pressure exerted by the vapour of. What Is Vapour Pressure Definition.
From ar.inspiredpencil.com
Vapor Pressure Example What Is Vapour Pressure Definition The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a. What Is Vapour Pressure Definition.
From www.geeksforgeeks.org
Vapour Pressure Definition, Raoult's Law, Formula & FAQs What Is Vapour Pressure Definition Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapor pressure is a property of a liquid based on the strength of its. The vapor pressure of. What Is Vapour Pressure Definition.
From www.brainkart.com
Vapour pressure of liquid Solutions Chemistry What Is Vapour Pressure Definition Vapor pressure is a property of a liquid based on the strength of its. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. That is, the pressure. What Is Vapour Pressure Definition.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapour Pressure Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure is a measure of the pressure (force per unit area). What Is Vapour Pressure Definition.
From www.worksheetsplanet.com
What is Pressure Definition of Pressure What Is Vapour Pressure Definition That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a property of a liquid based on the strength of its. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure of a. What Is Vapour Pressure Definition.
From www.youtube.com
What is Vapour Pressure? YouTube What Is Vapour Pressure Definition Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a property of a liquid based on the strength of its. The vapor pressure of a. What Is Vapour Pressure Definition.
From amariqoleon.blogspot.com
What is Vapor Pressure AmariqoLeon What Is Vapour Pressure Definition Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapor pressure is a property of a liquid based on the strength of its. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. That is, the pressure. What Is Vapour Pressure Definition.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapour Pressure Definition Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form,. What Is Vapour Pressure Definition.
From www.slideserve.com
PPT Vapour Pressure and Heat PowerPoint Presentation, free download What Is Vapour Pressure Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. What Is Vapour Pressure Definition.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube What Is Vapour Pressure Definition Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form,. What Is Vapour Pressure Definition.
From www.youtube.com
What is Vapour Pressure Vapour Pressure explained in hindi वाष्प What Is Vapour Pressure Definition That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a property of a liquid based on the strength of its. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapour pressure, pressure exerted by a vapour when the vapour. What Is Vapour Pressure Definition.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 What Is Vapour Pressure Definition Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. What Is Vapour Pressure Definition.
From electricalworkbook.com
What is Vapour Pressure Thermometer? Working Principle & Explanation What Is Vapour Pressure Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure is a measure of the pressure (force per unit area). What Is Vapour Pressure Definition.
From chemistryskills.com
Define and explain vapour pressure Chemistry Skills What Is Vapour Pressure Definition Vapor pressure is a property of a liquid based on the strength of its. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The vapor pressure is a measure of the pressure (force. What Is Vapour Pressure Definition.
From study.com
Vapor Pressure Definition, Equation & Examples Video & Lesson What Is Vapour Pressure Definition Vapor pressure is a property of a liquid based on the strength of its. The vapor pressure is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The vapor pressure of. What Is Vapour Pressure Definition.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is Vapour Pressure Definition Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. What Is Vapour Pressure Definition.