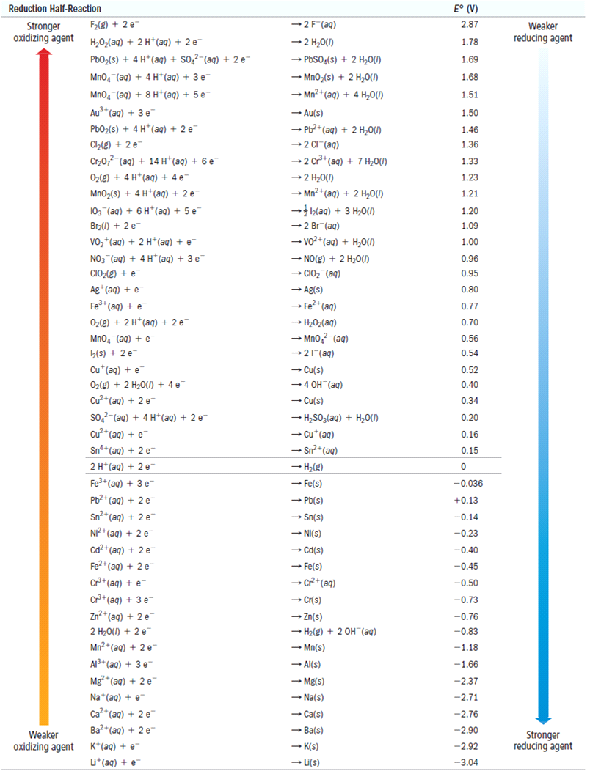
Standard Electrode Potential At 298 K . under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. some standard electrode potentials at 298 k are given below: P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. 45 rows standard electrode potentials in aqueous solution at 25°c. Interpret electrode potentials in terms of relative. describe and relate the definitions of electrode and cell potentials. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The symbol ‘e ocell ’ represents the standard electrode potential of a cell.
from parteybosa.blogspot.com
Interpret electrode potentials in terms of relative. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. 45 rows standard electrode potentials in aqueous solution at 25°c. in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. describe and relate the definitions of electrode and cell potentials. P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. some standard electrode potentials at 298 k are given below: the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:.
Standard Electrode Potential Table JEE Main Electrochemistry Part4
Standard Electrode Potential At 298 K describe and relate the definitions of electrode and cell potentials. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative. in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. some standard electrode potentials at 298 k are given below: describe and relate the definitions of electrode and cell potentials. P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. 45 rows standard electrode potentials in aqueous solution at 25°c.
From www.youtube.com
Some standard electrode potentials at \( 298 \mathrm{K} \) are below Standard Electrode Potential At 298 K some standard electrode potentials at 298 k are given below: The symbol ‘e ocell ’ represents the standard electrode potential of a cell. describe and relate the definitions of electrode and cell potentials. P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. the nernst equation is an equation relating. Standard Electrode Potential At 298 K.
From gioorxtcj.blob.core.windows.net
Standard Electrode Potential Of Nhe At 298 K Is at Olivia Nichols blog Standard Electrode Potential At 298 K describe and relate the definitions of electrode and cell potentials. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows. Standard Electrode Potential At 298 K.
From schools.aglasem.com
CBSE Notes Class 12 Chemistry Electrochemistry Standard Electrode Potential At 298 K some standard electrode potentials at 298 k are given below: under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. Interpret electrode potentials in terms of relative. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. in this work, standard. Standard Electrode Potential At 298 K.
From www.studypool.com
SOLUTION The standard electrode potential at 298 k Studypool Standard Electrode Potential At 298 K P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. describe and relate the definitions of electrode and cell potentials. some standard electrode potentials at 298 k are given below: Interpret electrode potentials in terms of relative. . Standard Electrode Potential At 298 K.
From askfilo.com
dilution at 298 K. Calculate the electrode potential. [EZn2+/Zn∘ =−0.76 V.. Standard Electrode Potential At 298 K Interpret electrode potentials in terms of relative. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. some standard electrode potentials at 298 k are given below: in this work, standard electrode potentials. Standard Electrode Potential At 298 K.
From gioorxtcj.blob.core.windows.net
Standard Electrode Potential Of Nhe At 298 K Is at Olivia Nichols blog Standard Electrode Potential At 298 K The symbol ‘e ocell ’ represents the standard electrode potential of a cell. Interpret electrode potentials in terms of relative. some standard electrode potentials at 298 k are given below: 45 rows standard electrode potentials in aqueous solution at 25°c. P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. . Standard Electrode Potential At 298 K.
From www.toppr.com
The standard reduction potential of the Ag^⊕ IAg electrode at 298 K is Standard Electrode Potential At 298 K 45 rows standard electrode potentials in aqueous solution at 25°c. the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. under standard conditions,. Standard Electrode Potential At 298 K.
From www.semanticscholar.org
Table 2 from Standard Electrode Potentials and Temperature Coefficients Standard Electrode Potential At 298 K The symbol ‘e ocell ’ represents the standard electrode potential of a cell. Interpret electrode potentials in terms of relative. the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one. Standard Electrode Potential At 298 K.
From pt.scribd.com
Standard Electrode and Reduction Potentials at 298 K Printable Sets Standard Electrode Potential At 298 K 45 rows standard electrode potentials in aqueous solution at 25°c. some standard electrode potentials at 298 k are given below: describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative. in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs.. Standard Electrode Potential At 298 K.
From www.doubtnut.com
The standard electrode potentials at 298 K are given below E(Zn^(2+) Standard Electrode Potential At 298 K Interpret electrode potentials in terms of relative. describe and relate the definitions of electrode and cell potentials. P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any. Standard Electrode Potential At 298 K.
From mungfali.com
Standard Electrode Potential Table Standard Electrode Potential At 298 K in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. Interpret electrode potentials in terms of relative. describe and relate the definitions of electrode and cell potentials. 45 rows standard electrode potentials in aqueous solution at 25°c. some standard electrode potentials at 298 k are given below:. Standard Electrode Potential At 298 K.
From www.youtube.com
NEET2023 At 298 K, the standard electrode potentials ofCu2+ / Cu Standard Electrode Potential At 298 K The symbol ‘e ocell ’ represents the standard electrode potential of a cell. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. Interpret electrode potentials in terms of relative. describe and relate the definitions of electrode and cell potentials. 45 rows standard electrode. Standard Electrode Potential At 298 K.
From askfilo.com
Table The Standard Electrode Potentials at 298 K Redox Reactions 57 Li+.. Standard Electrode Potential At 298 K the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. 45 rows standard electrode potentials in aqueous solution at 25°c. Interpret electrode potentials in. Standard Electrode Potential At 298 K.
From www.doubtnut.com
The standard electrode potentials at 298 K are given below E(Zn^(2+) Standard Electrode Potential At 298 K in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k,. Standard Electrode Potential At 298 K.
From www.numerade.com
SOLVEDCalculate the standard electrode potential for a cell in which Standard Electrode Potential At 298 K 45 rows standard electrode potentials in aqueous solution at 25°c. the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. in this work,. Standard Electrode Potential At 298 K.
From askfilo.com
Section A (Chemistry) 51. At 298 K, the standard electrode potentials o.. Standard Electrode Potential At 298 K the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative. Standard Electrode Potential At 298 K.
From parteybosa.blogspot.com
Standard Electrode Potential Table JEE Main Electrochemistry Part4 Standard Electrode Potential At 298 K The symbol ‘e ocell ’ represents the standard electrode potential of a cell. describe and relate the definitions of electrode and cell potentials. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. 372 rows the data below tabulates standard electrode potentials (e °),. Standard Electrode Potential At 298 K.
From www.youtube.com
JEE ADVANCED 2021 SOLUTION Some standard electrode potentials at 298 Standard Electrode Potential At 298 K some standard electrode potentials at 298 k are given below: the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. Interpret electrode potentials in. Standard Electrode Potential At 298 K.
From askfilo.com
Table 2.1 Standard Electrode Potentials at 298 K Filo Standard Electrode Potential At 298 K describe and relate the definitions of electrode and cell potentials. 45 rows standard electrode potentials in aqueous solution at 25°c. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. in this work, standard electrode potentials and temperature coefficients in water at 298.15. Standard Electrode Potential At 298 K.
From www.chegg.com
Solved Standard Reduction Potentials at 298 K Standard Standard Electrode Potential At 298 K The symbol ‘e ocell ’ represents the standard electrode potential of a cell. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. 45 rows standard electrode potentials in aqueous solution at 25°c. some standard electrode potentials at 298 k are given below: . Standard Electrode Potential At 298 K.
From www.numerade.com
SOLVEDThe standard reduction potential of the Ag^+ / Ag electrode at Standard Electrode Potential At 298 K in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. some standard electrode potentials at 298 k are given below: Interpret electrode potentials in terms of relative. describe and relate the definitions of electrode and cell potentials. under standard conditions, the standard electrode potential occurs in an. Standard Electrode Potential At 298 K.
From question.pandai.org
Standard Electrode Potential Standard Electrode Potential At 298 K the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative. Standard Electrode Potential At 298 K.
From askfilo.com
74. At 298 K, the standard electrode potentials of Cu2+/Cu,Zn2+/Zn,Fe2+/F.. Standard Electrode Potential At 298 K 45 rows standard electrode potentials in aqueous solution at 25°c. P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. under standard conditions, the standard electrode potential occurs in. Standard Electrode Potential At 298 K.
From www.toppr.com
Write Nernst equation for single electrode potential at 298 K Standard Electrode Potential At 298 K in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative. 45 rows standard electrode potentials in aqueous solution at 25°c.. Standard Electrode Potential At 298 K.
From askfilo.com
271 Table 8.1 The Standard Electrode Potentials at 298 K lons are present.. Standard Electrode Potential At 298 K some standard electrode potentials at 298 k are given below: The symbol ‘e ocell ’ represents the standard electrode potential of a cell. Interpret electrode potentials in terms of relative. describe and relate the definitions of electrode and cell potentials. in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on. Standard Electrode Potential At 298 K.
From askfilo.com
Q3 (Multiple Correct)Mark ItSome standard electrode potentials at 298 K.. Standard Electrode Potential At 298 K in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e. Standard Electrode Potential At 298 K.
From www.youtube.com
The standard potentials at `298 K` for the following halfreactions are Standard Electrode Potential At 298 K in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. the nernst equation is an equation relating the capacity. Standard Electrode Potential At 298 K.
From www.chegg.com
Solved Standard Reduction Potentials at 25°C (298 K) for Standard Electrode Potential At 298 K describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. the nernst equation is. Standard Electrode Potential At 298 K.
From mungfali.com
Table Of Standard Electrode Potentials Standard Electrode Potential At 298 K 45 rows standard electrode potentials in aqueous solution at 25°c. Interpret electrode potentials in terms of relative. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. some standard electrode potentials. Standard Electrode Potential At 298 K.
From askfilo.com
At 298 K, the standard electrode potentials of Cu2+/Cu,Zn2+/Zn,Fe2+/Fe a.. Standard Electrode Potential At 298 K P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. Interpret electrode potentials in terms of relative. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. . Standard Electrode Potential At 298 K.
From www.doubtnut.com
Some standard electrode potentials at 298K are given below. Cu^(2+)//C Standard Electrode Potential At 298 K some standard electrode potentials at 298 k are given below: describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative. 45 rows standard electrode potentials in aqueous solution at 25°c. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. in this work, standard electrode potentials. Standard Electrode Potential At 298 K.
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained Electrode Potential (Introduction) Standard Electrode Potential At 298 K P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. 45 rows standard electrode potentials in aqueous solution at 25°c. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. some standard electrode potentials at 298 k are given below: describe and relate the definitions of electrode. Standard Electrode Potential At 298 K.
From askfilo.com
64 At 298 K, the standard electrode potentials NEET of Cu2+/Cu,Zn2+/Zn,Fe.. Standard Electrode Potential At 298 K the nernst equation is an equation relating the capacity of an atom/ion to take up one or more electrons (reduction potential) measured at any conditions to that measured at standard conditions (standard reduction potentials) of 298 k and one molar or one atmospheric pressure. in this work, standard electrode potentials and temperature coefficients in water at 298.15 k,. Standard Electrode Potential At 298 K.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Standard Electrode Potential At 298 K in this work, standard electrode potentials and temperature coefficients in water at 298.15 k, based primarily on the nbs. P b2+/p b −0.13 v n i2+/n i −0.24 v cd2+/cd −0.40 v f e2+/f e −0.44. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The. Standard Electrode Potential At 298 K.
From www.doubtnut.com
The standard reduction potential of the Ag^(+)//Ag electrode at 298 K Standard Electrode Potential At 298 K describe and relate the definitions of electrode and cell potentials. The symbol ‘e ocell ’ represents the standard electrode potential of a cell. under standard conditions, the standard electrode potential occurs in an electrochemical cell say the temperature = 298k, pressure = 1atm, concentration = 1m. in this work, standard electrode potentials and temperature coefficients in water. Standard Electrode Potential At 298 K.