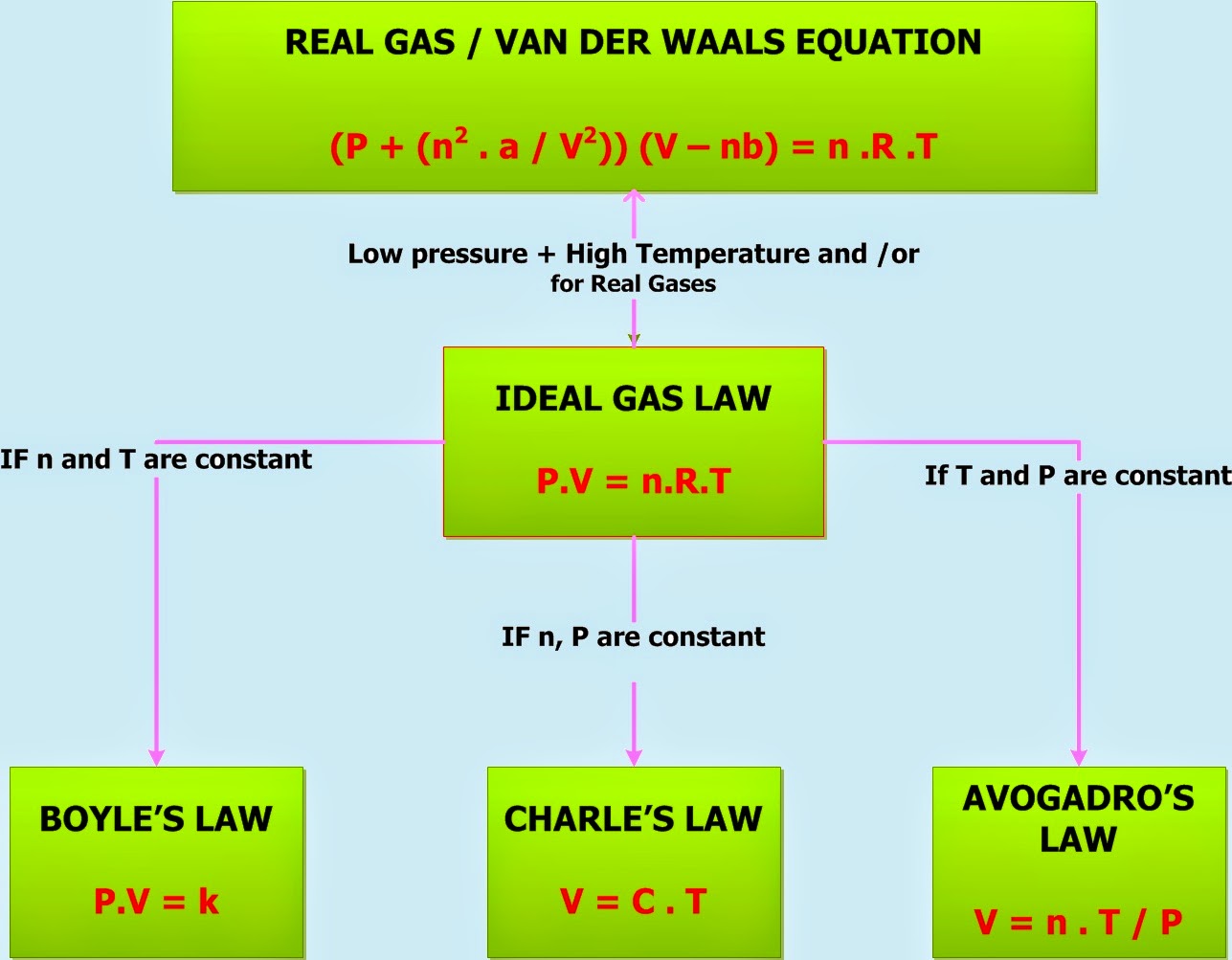
Ideal Gas Law And Airbags . The first widespread deployment systems used sodium azide to inflate airbags. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. In this experiment, you will be using the ideal gas law to predict the volume of gas produced from a simple chemical reaction. As airbags deploy, they fill quickly with the right kinds of gases to. The pressure used in the ideal gas equation is absolute pressure. Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). The ideal gas law says the two sides of the equation have to balance; Ideal gas laws are responsible for the working mechanics of airbags. The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. Adding moles of nitrogen gas forces the volume of the system.
from printablelibcoburg.z13.web.core.windows.net
Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). In this experiment, you will be using the ideal gas law to predict the volume of gas produced from a simple chemical reaction. The pressure used in the ideal gas equation is absolute pressure. The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. The first widespread deployment systems used sodium azide to inflate airbags. Ideal gas laws are responsible for the working mechanics of airbags. As airbags deploy, they fill quickly with the right kinds of gases to. Adding moles of nitrogen gas forces the volume of the system. The ideal gas law says the two sides of the equation have to balance; A sensor triggers a device that ignites the sodium azide, producing nitrogen gas.
Ideal Gas Law Explained
Ideal Gas Law And Airbags As airbags deploy, they fill quickly with the right kinds of gases to. Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). As airbags deploy, they fill quickly with the right kinds of gases to. Ideal gas laws are responsible for the working mechanics of airbags. Adding moles of nitrogen gas forces the volume of the system. The pressure used in the ideal gas equation is absolute pressure. The first widespread deployment systems used sodium azide to inflate airbags. The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. The ideal gas law says the two sides of the equation have to balance; In this experiment, you will be using the ideal gas law to predict the volume of gas produced from a simple chemical reaction.
From scienceinfo.com
Ideal Gas Equation Ideal Gas and Laws Ideal Gas Law And Airbags The pressure used in the ideal gas equation is absolute pressure. Adding moles of nitrogen gas forces the volume of the system. In this experiment, you will be using the ideal gas law to predict the volume of gas produced from a simple chemical reaction. As airbags deploy, they fill quickly with the right kinds of gases to. The ideal. Ideal Gas Law And Airbags.
From www.youtube.com
Gas Stoichiometry How does an airbag work? YouTube Ideal Gas Law And Airbags The pressure used in the ideal gas equation is absolute pressure. Adding moles of nitrogen gas forces the volume of the system. The ideal gas law says the two sides of the equation have to balance; The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved. Ideal Gas Law And Airbags.
From gaspinsami.blogspot.com
Gas Ideal Gas Law Ideal Gas Law And Airbags The first widespread deployment systems used sodium azide to inflate airbags. The pressure used in the ideal gas equation is absolute pressure. The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. In this experiment, you will be using the ideal gas law to predict. Ideal Gas Law And Airbags.
From www.lessonplanet.com
The Ideal Gas Law Instructional Video for 9th Higher Ed Lesson Ideal Gas Law And Airbags Adding moles of nitrogen gas forces the volume of the system. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. The ideal gas law says the two sides of the equation have to balance; As airbags deploy, they fill quickly with the right kinds of gases to. Automobile air bags are inflated with nitrogen gas, which. Ideal Gas Law And Airbags.
From www.youtube.com
Master the Ideal Gas Law in Chemistry A StepbyStep Guide [1510 Ideal Gas Law And Airbags The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. Adding moles of nitrogen gas forces the volume of the system. As airbags deploy, they fill quickly with the right kinds of gases to. The pressure used in the ideal gas equation is absolute pressure.. Ideal Gas Law And Airbags.
From materialmagicdeleon.z4.web.core.windows.net
Ideal Gas Law Sheet Ideal Gas Law And Airbags Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). Adding moles of nitrogen gas forces the volume of the system. In this experiment, you will be using the ideal gas law to predict the volume of gas produced from a simple chemical reaction. The pressure used in the ideal. Ideal Gas Law And Airbags.
From www.pearson.com
5 Ideal Gas Law Experiments PV=nRT or PV=NkT Pearson+ Channels Ideal Gas Law And Airbags Ideal gas laws are responsible for the working mechanics of airbags. Adding moles of nitrogen gas forces the volume of the system. The ideal gas law says the two sides of the equation have to balance; The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved. Ideal Gas Law And Airbags.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4387987 Ideal Gas Law And Airbags The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. The first widespread deployment systems used sodium azide to inflate airbags. The pressure used in the ideal gas equation is absolute pressure. The ideal gas law says the two sides of the equation have to. Ideal Gas Law And Airbags.
From www.pinterest.com
Gas Stoichiometry Airbag Lab Middle school science resources, Science Ideal Gas Law And Airbags Adding moles of nitrogen gas forces the volume of the system. The pressure used in the ideal gas equation is absolute pressure. In this experiment, you will be using the ideal gas law to predict the volume of gas produced from a simple chemical reaction. Ideal gas laws are responsible for the working mechanics of airbags. Automobile air bags are. Ideal Gas Law And Airbags.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Ideal Gas Law And Airbags The first widespread deployment systems used sodium azide to inflate airbags. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). In this experiment, you will be using the ideal gas law to predict the volume of. Ideal Gas Law And Airbags.
From plantecuador.com
Ideal Gas Law — Overview & Calculations Expii, ideal gas Ideal Gas Law And Airbags The pressure used in the ideal gas equation is absolute pressure. Adding moles of nitrogen gas forces the volume of the system. The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. As airbags deploy, they fill quickly with the right kinds of gases to.. Ideal Gas Law And Airbags.
From pitstopweekly.com
How Airbag Works? Pitstop Ideal Gas Law And Airbags The pressure used in the ideal gas equation is absolute pressure. The ideal gas law says the two sides of the equation have to balance; The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. A sensor triggers a device that ignites the sodium azide,. Ideal Gas Law And Airbags.
From studylib.net
12.2 Ideal Gasses and the Ideal Gas Law Ideal Gas Law And Airbags Ideal gas laws are responsible for the working mechanics of airbags. The first widespread deployment systems used sodium azide to inflate airbags. In this experiment, you will be using the ideal gas law to predict the volume of gas produced from a simple chemical reaction. Adding moles of nitrogen gas forces the volume of the system. The effective operation of. Ideal Gas Law And Airbags.
From mmerevise.co.uk
The Ideal Gas Equation MME Ideal Gas Law And Airbags Ideal gas laws are responsible for the working mechanics of airbags. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. The ideal gas law says the two sides of the equation have to balance; Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). Adding moles. Ideal Gas Law And Airbags.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 Ideal Gas Law And Airbags The first widespread deployment systems used sodium azide to inflate airbags. Adding moles of nitrogen gas forces the volume of the system. As airbags deploy, they fill quickly with the right kinds of gases to. Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). In this experiment, you will. Ideal Gas Law And Airbags.
From www.chemistrylearner.com
Ideal Gas Law Statement, Characteristics, Formula & Problems Ideal Gas Law And Airbags The pressure used in the ideal gas equation is absolute pressure. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. Adding moles of nitrogen gas forces the volume of the system. As airbags deploy, they fill quickly with the right kinds of gases to. The ideal gas law says the two sides of the equation have. Ideal Gas Law And Airbags.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica Ideal Gas Law And Airbags The ideal gas law says the two sides of the equation have to balance; Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). The pressure used in the ideal gas equation is absolute pressure. The effective operation of an airbag requires that it be rapidly inflated with an appropriate. Ideal Gas Law And Airbags.
From erebusryling.blogspot.com
Ideal Gas Law R Values PPT Gas Laws PowerPoint Presentation ID Ideal Gas Law And Airbags Adding moles of nitrogen gas forces the volume of the system. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. The first widespread deployment systems used sodium azide to inflate airbags. Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). Ideal gas laws are responsible. Ideal Gas Law And Airbags.
From www.youtube.com
SCIENCE BEHIND AIRBAGS (IDEAL GAS LAW) YouTube Ideal Gas Law And Airbags Ideal gas laws are responsible for the working mechanics of airbags. The first widespread deployment systems used sodium azide to inflate airbags. As airbags deploy, they fill quickly with the right kinds of gases to. The pressure used in the ideal gas equation is absolute pressure. Adding moles of nitrogen gas forces the volume of the system. A sensor triggers. Ideal Gas Law And Airbags.
From www.youtube.com
Introduction to the Ideal Gas Law Formula YouTube Ideal Gas Law And Airbags Ideal gas laws are responsible for the working mechanics of airbags. The ideal gas law says the two sides of the equation have to balance; The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. The pressure used in the ideal gas equation is absolute. Ideal Gas Law And Airbags.
From www.studocu.com
Gas Laws Worksheet answer key Gas Laws Worksheet atm = 760 mm Hg Ideal Gas Law And Airbags A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. The ideal gas law says the two sides of the equation have to balance; The first widespread deployment systems used sodium azide to inflate airbags. The pressure used in the ideal gas equation is absolute pressure. Adding moles of nitrogen gas forces the volume of the system.. Ideal Gas Law And Airbags.
From printablelibcoburg.z13.web.core.windows.net
Ideal Gas Law Explained Ideal Gas Law And Airbags The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. As airbags deploy, they fill quickly with the right kinds of gases to. Ideal gas laws are responsible for the working mechanics of airbags. In this experiment, you will be using the ideal gas law. Ideal Gas Law And Airbags.
From www.scribd.com
Ideal Gas Law 2 PDF Ideal Gas Law And Airbags The pressure used in the ideal gas equation is absolute pressure. In this experiment, you will be using the ideal gas law to predict the volume of gas produced from a simple chemical reaction. The first widespread deployment systems used sodium azide to inflate airbags. Adding moles of nitrogen gas forces the volume of the system. The ideal gas law. Ideal Gas Law And Airbags.
From sciencenotes.org
Ideal Gas Law Formula and Examples Ideal Gas Law And Airbags The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. The pressure used in the ideal gas equation is absolute pressure. The first widespread deployment systems used sodium azide to inflate airbags. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas.. Ideal Gas Law And Airbags.
From energyeducation.ca
Ideal gas law Energy Education Ideal Gas Law And Airbags A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. The ideal gas law says the two sides of the equation have to balance; The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. In this experiment, you will be using the. Ideal Gas Law And Airbags.
From slideplayer.com
Before Bell Rings Chill out. ppt download Ideal Gas Law And Airbags The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. The pressure used in the ideal gas equation is absolute pressure. Automobile air bags are inflated with nitrogen gas, which is formed. Ideal Gas Law And Airbags.
From gaspinsami.blogspot.com
Gas What Is The Ideal Gas Law Ideal Gas Law And Airbags The first widespread deployment systems used sodium azide to inflate airbags. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. Ideal gas laws are responsible for the working mechanics of airbags. As airbags deploy, they fill quickly with the right kinds of gases to. The effective operation of an airbag requires that it be rapidly inflated. Ideal Gas Law And Airbags.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 Ideal Gas Law And Airbags The first widespread deployment systems used sodium azide to inflate airbags. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. As airbags deploy, they fill quickly with the right kinds of gases to. The ideal gas law says the two sides of the equation have to balance; The pressure used in the ideal gas equation is. Ideal Gas Law And Airbags.
From www.youtube.com
The Ideal Gas Law YouTube Ideal Gas Law And Airbags The pressure used in the ideal gas equation is absolute pressure. The first widespread deployment systems used sodium azide to inflate airbags. The ideal gas law says the two sides of the equation have to balance; The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved. Ideal Gas Law And Airbags.
From www.shutterstock.com
8,299 Gas law Images, Stock Photos & Vectors Shutterstock Ideal Gas Law And Airbags The ideal gas law says the two sides of the equation have to balance; The pressure used in the ideal gas equation is absolute pressure. Adding moles of nitrogen gas forces the volume of the system. The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved. Ideal Gas Law And Airbags.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID5758540 Ideal Gas Law And Airbags Adding moles of nitrogen gas forces the volume of the system. The first widespread deployment systems used sodium azide to inflate airbags. Ideal gas laws are responsible for the working mechanics of airbags. The ideal gas law says the two sides of the equation have to balance; The effective operation of an airbag requires that it be rapidly inflated with. Ideal Gas Law And Airbags.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Ideal Gas Law And Airbags Adding moles of nitrogen gas forces the volume of the system. As airbags deploy, they fill quickly with the right kinds of gases to. The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in. The first widespread deployment systems used sodium azide to inflate airbags.. Ideal Gas Law And Airbags.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Ideal Gas Law And Airbags Ideal gas laws are responsible for the working mechanics of airbags. The pressure used in the ideal gas equation is absolute pressure. Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). The first widespread deployment systems used sodium azide to inflate airbags. A sensor triggers a device that ignites. Ideal Gas Law And Airbags.
From studylib.net
Ideal Gas Law Ideal Gas Law And Airbags The first widespread deployment systems used sodium azide to inflate airbags. A sensor triggers a device that ignites the sodium azide, producing nitrogen gas. Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). The pressure used in the ideal gas equation is absolute pressure. As airbags deploy, they fill. Ideal Gas Law And Airbags.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID352184 Ideal Gas Law And Airbags The pressure used in the ideal gas equation is absolute pressure. Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan 3). Adding moles of nitrogen gas forces the volume of the system. The ideal gas law says the two sides of the equation have to balance; A sensor triggers a. Ideal Gas Law And Airbags.