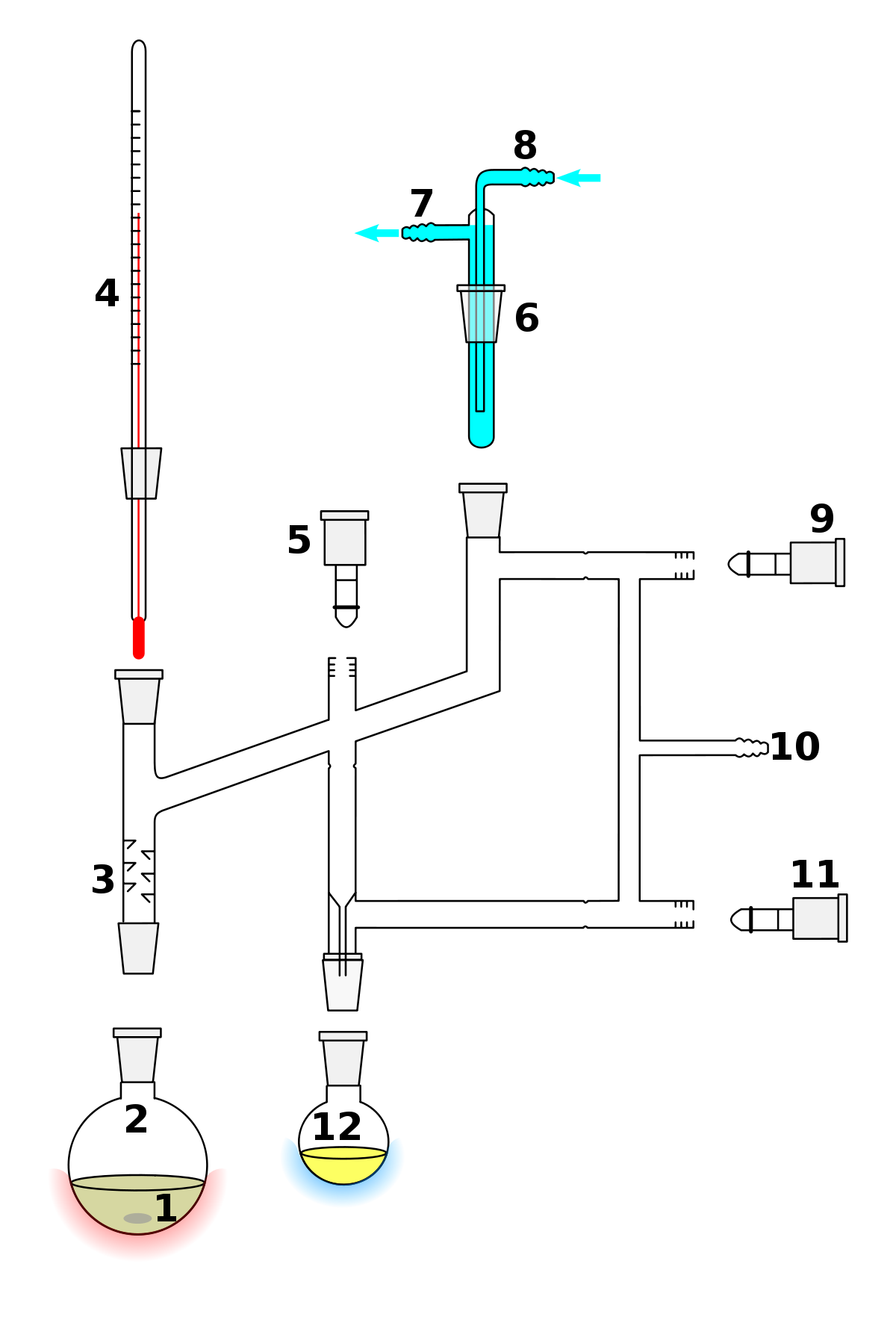
What Is Meant By Vacuum Distillation . The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Learn what is vacuum distillation, how it works, and when it is used in the chemical industry. Vacuum distillation is a technique for the purification or separation of liquids or solvents. The condensate, also called distillate, can be reused. Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation.
from www.theengineeringconcepts.com
Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. The condensate, also called distillate, can be reused. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Learn what is vacuum distillation, how it works, and when it is used in the chemical industry. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities.
Types of Distillation The Engineering Concepts
What Is Meant By Vacuum Distillation A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. The condensate, also called distillate, can be reused. The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Learn what is vacuum distillation, how it works, and when it is used in the chemical industry. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form What Is Meant By Vacuum Distillation Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. The wastewater. What Is Meant By Vacuum Distillation.
From www.simplepharmanotes.com
Distillation under reduced pressure / Vacuum Distillation. What Is Meant By Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to. What Is Meant By Vacuum Distillation.
From www.pressurecontrolsolutions.com
Vacuum Distillation issues? Call Pressure Control Solutions! What Is Meant By Vacuum Distillation Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. Learn what. What Is Meant By Vacuum Distillation.
From thepetrosolutions.com
Vacuum Distillation Unit in Oil Refinery The Petro Solutions What Is Meant By Vacuum Distillation Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. The condensate, also called distillate, can be reused. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too. What Is Meant By Vacuum Distillation.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form What Is Meant By Vacuum Distillation Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. Learn what is vacuum distillation, how it works, and when it is used in the chemical industry. Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. The. What Is Meant By Vacuum Distillation.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry What Is Meant By Vacuum Distillation The condensate, also called distillate, can be reused. The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Learn what is. What Is Meant By Vacuum Distillation.
From www.processingmagazine.com
Controlling chemical vacuum processes with direct sealing diaphragm What Is Meant By Vacuum Distillation The condensate, also called distillate, can be reused. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Learn. What Is Meant By Vacuum Distillation.
From chemicaltweak.com
When Vacuum Distillation Is Selected Application And Uses Of VDU What Is Meant By Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Learn how to perform a vacuum distillation using a simple or. What Is Meant By Vacuum Distillation.
From www.researchgate.net
Scheme of the vacuum distillation for catalyst recycle 2.3 Preparation What Is Meant By Vacuum Distillation The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. Vacuum distillation is a technique for the purification or. What Is Meant By Vacuum Distillation.
From foodtechnotes.com
Distillation Principle and Types Food Tech Notes What Is Meant By Vacuum Distillation Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Learn what is vacuum distillation,. What Is Meant By Vacuum Distillation.
From onlinelibrary.wiley.com
Simulation of vacuum distillation to produce alcohol‐free beer What Is Meant By Vacuum Distillation The condensate, also called distillate, can be reused. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than. What Is Meant By Vacuum Distillation.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts What Is Meant By Vacuum Distillation The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. The condensate, also called distillate, can be reused. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Learn how. What Is Meant By Vacuum Distillation.
From www.youtube.com
Distillation Process Vacuum Distillation Process Distillation What Is Meant By Vacuum Distillation Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. Learn. What Is Meant By Vacuum Distillation.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog What Is Meant By Vacuum Distillation Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Vacuum distillation is a method. What Is Meant By Vacuum Distillation.
From blog.digivac.com
3 Benefits of Vacuum Distillation in Low and Medium Vacuum What Is Meant By Vacuum Distillation A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Find out how to create and break vacuum in distillation columns. What Is Meant By Vacuum Distillation.
From www.theengineersperspectives.com
Vacuum Distillation System The Engineer's Perspective What Is Meant By Vacuum Distillation Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Learn what is vacuum distillation, how it works, and when it is used in the chemical industry. The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Vacuum distillation is a. What Is Meant By Vacuum Distillation.
From www.chemistry-online.com
Vacuum distillation Chemistry Online What Is Meant By Vacuum Distillation Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Find out how to create and break vacuum in distillation columns and reactors, and. What Is Meant By Vacuum Distillation.
From www.youtube.com
Vacuum Distillation YouTube What Is Meant By Vacuum Distillation Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. A vacuum distillation is used when the boiling point of the compound (or the. What Is Meant By Vacuum Distillation.
From chemoconcept.blogspot.com
Vacuum Distillation Unit Part 3 Petroleum Refining Article Series What Is Meant By Vacuum Distillation The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound. What Is Meant By Vacuum Distillation.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts What Is Meant By Vacuum Distillation The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Find out how to create. What Is Meant By Vacuum Distillation.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry What Is Meant By Vacuum Distillation Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. Learn how. What Is Meant By Vacuum Distillation.
From www.researchgate.net
The schematic for the vacuum distillation process. Download What Is Meant By Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Find out how to create and break vacuum in. What Is Meant By Vacuum Distillation.
From www.researchgate.net
Schematic Process Flow Diagram for Vacuum Distillation Download What Is Meant By Vacuum Distillation The condensate, also called distillate, can be reused. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled. What Is Meant By Vacuum Distillation.
From www.youtube.com
Distillation under reduced pressure Vacuum Distillation What Is Meant By Vacuum Distillation Learn what is vacuum distillation, how it works, and when it is used in the chemical industry. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Find out how to. What Is Meant By Vacuum Distillation.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts What Is Meant By Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Learn what is vacuum distillation, how. What Is Meant By Vacuum Distillation.
From mavink.com
Vacuum Distillation Set Up What Is Meant By Vacuum Distillation Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents. The condensate, also called distillate, can be reused.. What Is Meant By Vacuum Distillation.
From www.researchgate.net
Diagram of the vacuum distillation still used for purification What Is Meant By Vacuum Distillation Learn what is vacuum distillation, how it works, and when it is used in the chemical industry. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil. What Is Meant By Vacuum Distillation.
From schlenklinesurvivalguide.com
Static Vacuum Distillation The Schlenk Line Survival Guide What Is Meant By Vacuum Distillation Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. Learn what is vacuum distillation, how it works, and when it is used in the chemical industry. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil. What Is Meant By Vacuum Distillation.
From thejapanesebar.com
Atmospheric and Vacuum Distillation of Shochu Joatsu, Genatsu What Is Meant By Vacuum Distillation Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the solvent off) without significant. Find. What Is Meant By Vacuum Distillation.
From www.researchgate.net
Vacuum distillation apparatus. Download Scientific Diagram What Is Meant By Vacuum Distillation The condensate, also called distillate, can be reused. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than. What Is Meant By Vacuum Distillation.
From www.researchgate.net
The vacuum distillation apparatus scheme. Download Scientific Diagram What Is Meant By Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. Find out how to create and break vacuum. What Is Meant By Vacuum Distillation.
From www.researchgate.net
Process flow diagram of Vacuum Distillation Unit Download Scientific What Is Meant By Vacuum Distillation Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. Vacuum distillation is a technique for the purification or separation of liquids or solvents. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in. What Is Meant By Vacuum Distillation.
From testbook.com
team Distillation Principle, Working, Advantages & Application What Is Meant By Vacuum Distillation The wastewater is evaporated, the dirt remains behind, the rising steam is free of impurities. Find out how to create and break vacuum in distillation columns and reactors, and see examples of crude oil distillation. Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor. The. What Is Meant By Vacuum Distillation.
From www.youtube.com
What is vacuum distillation use of vacuum distillation what is air What Is Meant By Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents. Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. The condensate, also called distillate, can be reused. A vacuum distillation is used when the boiling point of the compound (or the solvent) is. What Is Meant By Vacuum Distillation.
From www.researchgate.net
Vacuum Distillation Process to Produce Lubricants Download What Is Meant By Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Learn how to perform a vacuum distillation using a simple or fractional setup, with a water aspirator or a portable vacuum pump. The condensate, also called distillate, can be reused.. What Is Meant By Vacuum Distillation.