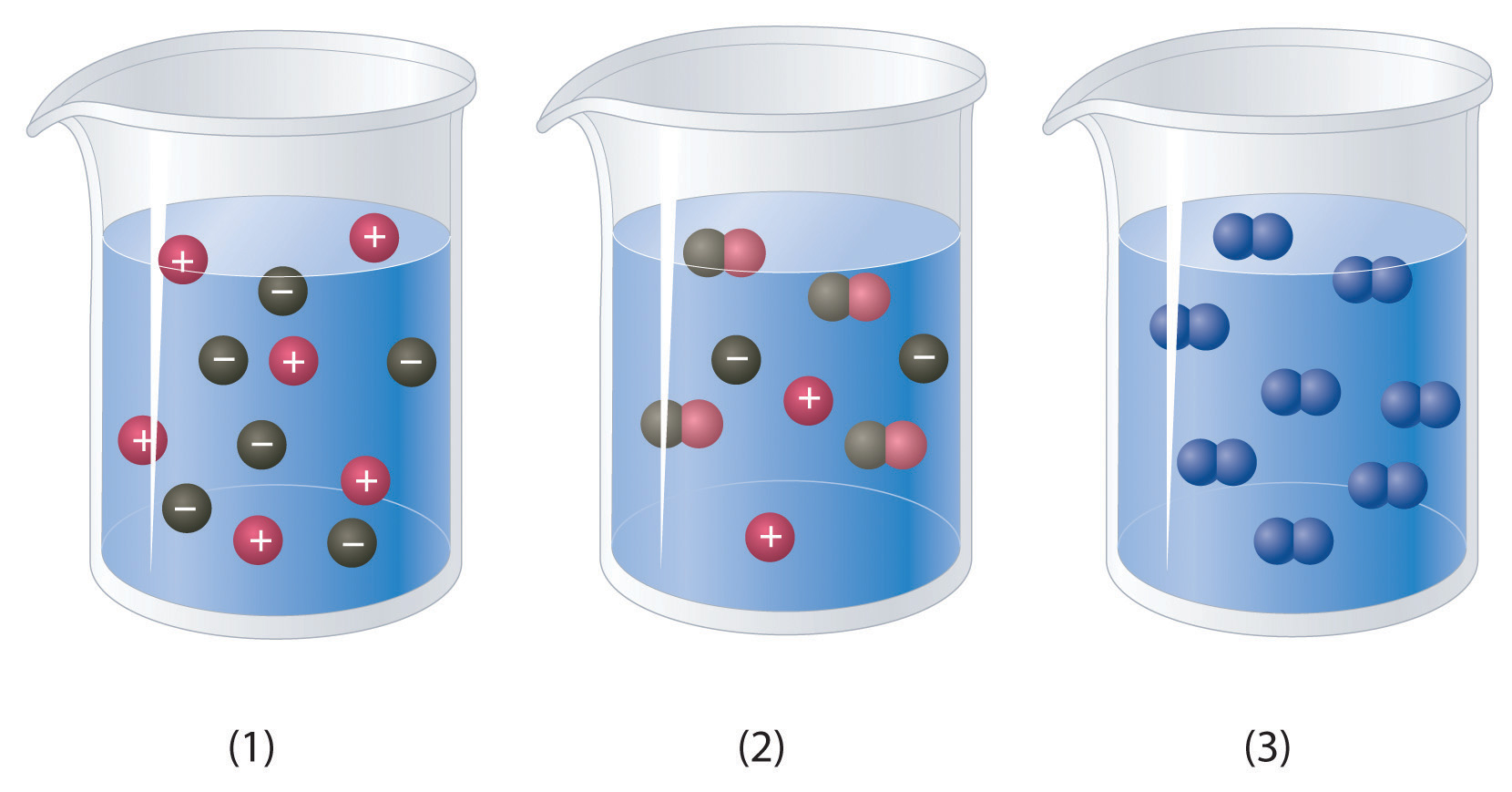
Example Of Gas Solvent And Solid Solute . The focus of chapter 15: Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Water was water's role in the formation of aqueous solutions. The same is true of liquids at temperatures well below their boiling points. The solvent is considered the major species of the solution, or the substance there is more of. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. There are seven main types of solutions, this list includes the types and examples: These two cases of gaseous solutions can be. The solute is the substance that is being dissolved, while the solvent is the dissolving medium. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. Solubility is a measure of how much of a solute will dissolve in a solvent. For example, the main solute in sea water is.
from www.wou.edu
These two cases of gaseous solutions can be. Water was water's role in the formation of aqueous solutions. There are seven main types of solutions, this list includes the types and examples: Solubility is a measure of how much of a solute will dissolve in a solvent. In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. For example, the main solute in sea water is. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. The focus of chapter 15: The solute is the substance that is being dissolved, while the solvent is the dissolving medium. The same is true of liquids at temperatures well below their boiling points.
CH150 Chapter 7 Solutions Chemistry
Example Of Gas Solvent And Solid Solute Water was water's role in the formation of aqueous solutions. Water was water's role in the formation of aqueous solutions. Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. The same is true of liquids at temperatures well below their boiling points. The solvent is considered the major species of the solution, or the substance there is more of. There are seven main types of solutions, this list includes the types and examples: These two cases of gaseous solutions can be. In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. The focus of chapter 15: The solute is the substance that is being dissolved, while the solvent is the dissolving medium. For example, the main solute in sea water is. Solubility is a measure of how much of a solute will dissolve in a solvent.
From www.slideserve.com
PPT Types of mixtures PowerPoint Presentation ID4695980 Example Of Gas Solvent And Solid Solute In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. The solvent is considered the major species of the solution, or the substance there is more of. Solubility is a measure of how much of a solute will dissolve in a solvent. These two cases. Example Of Gas Solvent And Solid Solute.
From www.youtube.com
Year 7 Science Lesson Solute, Solvent, Solution EdPlace YouTube Example Of Gas Solvent And Solid Solute In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. There are seven main types of solutions, this list includes the types and examples: A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. Solubility is a measure of how much. Example Of Gas Solvent And Solid Solute.
From www.youtube.com
Difference between solute and solvent YouTube Example Of Gas Solvent And Solid Solute A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Water was water's role in the formation of aqueous solutions. There are seven main types of solutions, this list includes the. Example Of Gas Solvent And Solid Solute.
From devinitionva.blogspot.com
Definition Of Solute Solvent And Solution DEFINITIONVA Example Of Gas Solvent And Solid Solute There are seven main types of solutions, this list includes the types and examples: The solute is the substance that is being dissolved, while the solvent is the dissolving medium. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. The same is true of liquids at temperatures well below their boiling points. A solution is. Example Of Gas Solvent And Solid Solute.
From slideplayer.com
Solutions. ppt download Example Of Gas Solvent And Solid Solute Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. For example, the main solute in sea water is. The solvent is considered the major species of the solution, or the substance there is more of. The same is true of liquids at temperatures well below their boiling points. In most of the solutions we will describe in. Example Of Gas Solvent And Solid Solute.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Example Of Gas Solvent And Solid Solute These two cases of gaseous solutions can be. The focus of chapter 15: The solute is the substance that is being dissolved, while the solvent is the dissolving medium. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Generally, polar solvents dissolve. Example Of Gas Solvent And Solid Solute.
From www.dreamstime.com
Dissolving Solids. Solubility Chemistry Stock Vector Illustration of Example Of Gas Solvent And Solid Solute Solubility is a measure of how much of a solute will dissolve in a solvent. These two cases of gaseous solutions can be. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. For example, the main solute in sea water is. In most. Example Of Gas Solvent And Solid Solute.
From www.youtube.com
Solution with examples Identify the solute and solvent YouTube Example Of Gas Solvent And Solid Solute For example, the main solute in sea water is. There are seven main types of solutions, this list includes the types and examples: The solute is the substance that is being dissolved, while the solvent is the dissolving medium. Solubility is a measure of how much of a solute will dissolve in a solvent. The solvent is considered the major. Example Of Gas Solvent And Solid Solute.
From laboratoryinfo.com
Difference between Solute and Solvent Example Of Gas Solvent And Solid Solute These two cases of gaseous solutions can be. The same is true of liquids at temperatures well below their boiling points. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. A solution is a homogeneous mixture consisting of a solute dissolved into. Example Of Gas Solvent And Solid Solute.
From mungfali.com
Solids Liquids Gases Chart Example Of Gas Solvent And Solid Solute A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. The solvent is considered the major species of the solution, or the substance there is more of. The same is true of liquids at temperatures well below their boiling points. There are seven. Example Of Gas Solvent And Solid Solute.
From slideplayer.com
Chemistry of Solutions ppt download Example Of Gas Solvent And Solid Solute For example, the main solute in sea water is. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. There are seven main types of solutions, this list includes the types and examples: Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve. Example Of Gas Solvent And Solid Solute.
From slideplayer.com
Unit 10 Solutions Lecture 2 Solutions and Solubility ppt download Example Of Gas Solvent And Solid Solute A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. These two cases of gaseous solutions can be. The focus of chapter 15: For example, the main solute in sea water is. The same is true of liquids at temperatures well below their boiling points. Solubility is a measure of how much of a solute will. Example Of Gas Solvent And Solid Solute.
From www.toppr.com
Two substances X and Y are dissolved in water under suitable conditions Example Of Gas Solvent And Solid Solute The focus of chapter 15: For example, the main solute in sea water is. These two cases of gaseous solutions can be. Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. The solute is the substance that is being dissolved, while the solvent is the dissolving medium. Solubility is a measure of how much of a solute. Example Of Gas Solvent And Solid Solute.
From chem.libretexts.org
Solubility and Precipitation Chemistry LibreTexts Example Of Gas Solvent And Solid Solute There are seven main types of solutions, this list includes the types and examples: These two cases of gaseous solutions can be. The solvent is considered the major species of the solution, or the substance there is more of. For example, the main solute in sea water is. A solution is made when a solute close solute the solid (or. Example Of Gas Solvent And Solid Solute.
From miraferspatel.blogspot.com
Which Word Describes a Solid That Cannot Dissolve in Water Example Of Gas Solvent And Solid Solute A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. The focus of chapter 15: For example, the main solute in sea water is. The solvent is considered the major species of the solution, or the substance there is more of. There are seven main types of solutions, this list includes the types and examples: Generally,. Example Of Gas Solvent And Solid Solute.
From www.animalia-life.club
Gas Solution Examples Chemistry Example Of Gas Solvent And Solid Solute Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. Solubility is a measure of how much of a solute will dissolve in a solvent. There are seven main types of solutions, this. Example Of Gas Solvent And Solid Solute.
From courses.lumenlearning.com
The Dissolution Process Chemistry Example Of Gas Solvent And Solid Solute The solute is the substance that is being dissolved, while the solvent is the dissolving medium. There are seven main types of solutions, this list includes the types and examples: In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. Water was water's role in. Example Of Gas Solvent And Solid Solute.
From www.studypool.com
SOLUTION Examples of solid liquid gas Studypool Example Of Gas Solvent And Solid Solute Water was water's role in the formation of aqueous solutions. Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. For example, the main solute in sea water is. The solvent is considered the major species of the solution, or the substance there is more of. A solution is a homogeneous mixture consisting of a solute dissolved into. Example Of Gas Solvent And Solid Solute.
From www.youtube.com
How to Calculate Mass Percent of Solute and Solvent of Solution Example Of Gas Solvent And Solid Solute Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Solubility is a measure of how much of a solute will dissolve in a solvent. In most of the solutions we. Example Of Gas Solvent And Solid Solute.
From www.difference101.com
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples Example Of Gas Solvent And Solid Solute A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. There are seven main types of solutions, this list includes the types and examples: The solute is the substance that is being dissolved, while the solvent is the dissolving medium. The solvent is. Example Of Gas Solvent And Solid Solute.
From studiousguy.com
8 Solute Examples in Everyday Life StudiousGuy Example Of Gas Solvent And Solid Solute Solubility is a measure of how much of a solute will dissolve in a solvent. For example, the main solute in sea water is. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. In most of the solutions we will describe in. Example Of Gas Solvent And Solid Solute.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Example Of Gas Solvent And Solid Solute The solute is the substance that is being dissolved, while the solvent is the dissolving medium. For example, the main solute in sea water is. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. The same is true of liquids at temperatures. Example Of Gas Solvent And Solid Solute.
From sites.google.com
9/8/15 Miss Day's Science Class Blog Example Of Gas Solvent And Solid Solute The same is true of liquids at temperatures well below their boiling points. Solubility is a measure of how much of a solute will dissolve in a solvent. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. The solvent is considered the major. Example Of Gas Solvent And Solid Solute.
From www.deperu.com
Diagrama infográfico de la solución que muestra el recipiente llenado Example Of Gas Solvent And Solid Solute The same is true of liquids at temperatures well below their boiling points. Solubility is a measure of how much of a solute will dissolve in a solvent. Water was water's role in the formation of aqueous solutions. For example, the main solute in sea water is. A solution is a homogeneous mixture consisting of a solute dissolved into a. Example Of Gas Solvent And Solid Solute.
From socratic.org
What is the relationship among solutions, solutes, and solvents? Socratic Example Of Gas Solvent And Solid Solute Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. For example, the main solute in sea water is. These two cases of gaseous solutions can be. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. There are seven main types of solutions, this list includes the types and examples: The focus of. Example Of Gas Solvent And Solid Solute.
From brunofuga.adv.br
Solution Chemistry Definition, Types, Examples, 52 OFF Example Of Gas Solvent And Solid Solute These two cases of gaseous solutions can be. Solubility is a measure of how much of a solute will dissolve in a solvent. The same is true of liquids at temperatures well below their boiling points. In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or. Example Of Gas Solvent And Solid Solute.
From 139.59.164.119
10 Examples of Solids, Liquids, Gases, and Plasma Example Of Gas Solvent And Solid Solute Water was water's role in the formation of aqueous solutions. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. The solute is the substance that is being dissolved, while the solvent is the dissolving medium. Solubility is a measure of how much of a solute will dissolve in a solvent. There are seven main types. Example Of Gas Solvent And Solid Solute.
From ar.inspiredpencil.com
Gas Examples For Kids Example Of Gas Solvent And Solid Solute The focus of chapter 15: In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. These two cases of gaseous solutions can be. Solubility is a measure of how much of a solute will dissolve in a solvent. The same is true of liquids at. Example Of Gas Solvent And Solid Solute.
From sciencenotes.org
Examples of Gases What Is a Gas? Example Of Gas Solvent And Solid Solute Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. The focus of chapter 15: In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. These two cases of gaseous solutions can be. Water was water's role in the formation of aqueous solutions.. Example Of Gas Solvent And Solid Solute.
From www.slideserve.com
PPT Revision PowerPoint Presentation, free download ID5491285 Example Of Gas Solvent And Solid Solute Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Solubility is a measure of how much of a solute will dissolve in a solvent. For example, the main solute in. Example Of Gas Solvent And Solid Solute.
From byjus.com
Draw a sketch to show the arrangement of particles in solid, liquid and Example Of Gas Solvent And Solid Solute Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. These two cases of gaseous solutions can be. The focus of chapter 15: The solvent is considered the major species of the solution, or the substance there is more of. The solute is the. Example Of Gas Solvent And Solid Solute.
From slideplayer.com
N39 Properties of Solutions ppt download Example Of Gas Solvent And Solid Solute Solubility is a measure of how much of a solute will dissolve in a solvent. The solute is the substance that is being dissolved, while the solvent is the dissolving medium. Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves. Example Of Gas Solvent And Solid Solute.
From www.pinterest.com
Introduction to Solutions Worksheet Set (Solutes, Solvents, and Example Of Gas Solvent And Solid Solute The solute is the substance that is being dissolved, while the solvent is the dissolving medium. The focus of chapter 15: Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. These two cases of gaseous solutions can be. Solubility is a measure of how much of a solute will dissolve in a solvent. There are seven main. Example Of Gas Solvent And Solid Solute.
From medium.com
What Is the Difference Between Solute And Solvent? by Diksha Bhardwaj Example Of Gas Solvent And Solid Solute The solvent is considered the major species of the solution, or the substance there is more of. In most of the solutions we will describe in this textbook, there will be no ambiguity about whether a component is the solvent or the. These two cases of gaseous solutions can be. For example, the main solute in sea water is. The. Example Of Gas Solvent And Solid Solute.
From www.slideserve.com
PPT What is a solution? PowerPoint Presentation, free download ID Example Of Gas Solvent And Solid Solute A solution is made when a solute close solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. These two cases of gaseous solutions can be. Water was water's role in the formation of aqueous solutions. Generally, polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Solubility is a. Example Of Gas Solvent And Solid Solute.