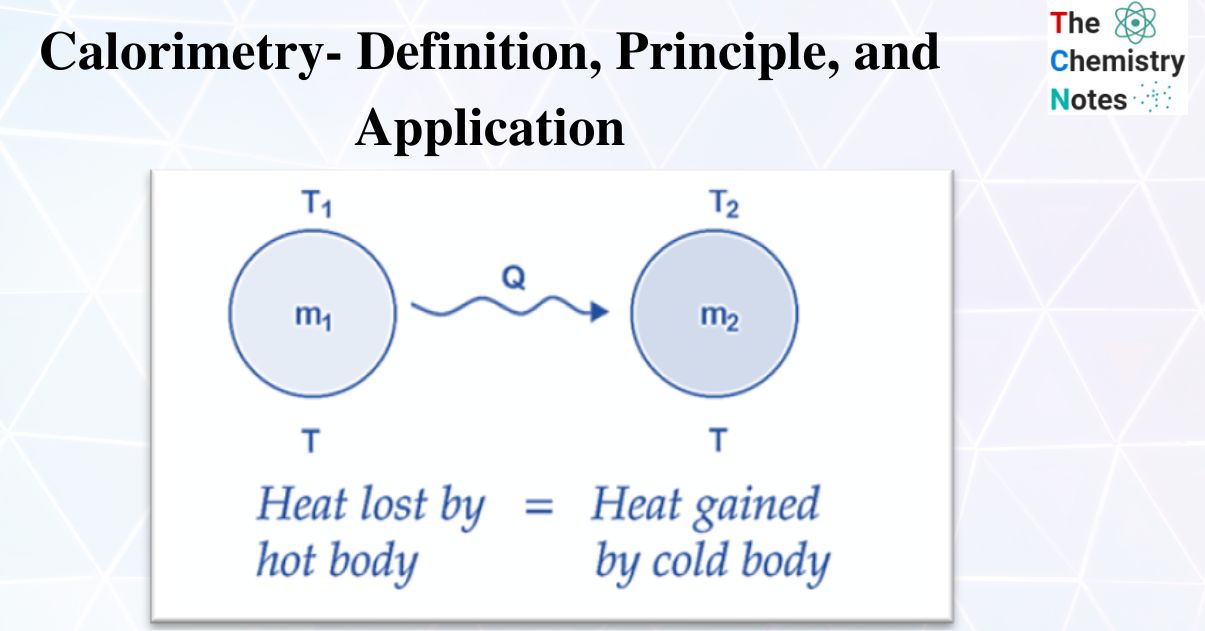
Calorimeter Principle And Application . When two bodies of different temperatures (preferably a solid and a liquid) are placed in. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Fundamentals, instrumentation and applications, first edition. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.
from thechemistrynotes.com
calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Fundamentals, instrumentation and applications, first edition. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. When two bodies of different temperatures (preferably a solid and a liquid) are placed in.
Calorimetry Definition, Principle, Types, Application, and Limitations
Calorimeter Principle And Application When two bodies of different temperatures (preferably a solid and a liquid) are placed in. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Fundamentals, instrumentation and applications, first edition. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. When two bodies of different temperatures (preferably a solid and a liquid) are placed in.
From library.achievingthedream.org
Calorimetry Introductory Chemistry Lecture & Lab Calorimeter Principle And Application calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Fundamentals, instrumentation and applications, first edition. a calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Principle And Application.
From byjus.com
State the Principle of calorimetry Calorimeter Principle And Application calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. When two. Calorimeter Principle And Application.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Principle And Application calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. Fundamentals, instrumentation and applications, first edition. a. Calorimeter Principle And Application.
From saylordotorg.github.io
Calorimetry Calorimeter Principle And Application When two bodies of different temperatures (preferably a solid and a liquid) are placed in. Fundamentals, instrumentation and applications, first edition. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimeter Principle And Application.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Principle And Application calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. Fundamentals, instrumentation and applications, first edition. a calorimeter. Calorimeter Principle And Application.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Principle And Application a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and. Calorimeter Principle And Application.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Principle And Application calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. Fundamentals, instrumentation and applications, first edition. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. . Calorimeter Principle And Application.
From momomadeleinejackson.blogspot.com
differential scanning calorimetry principle Madeleine Jackson Calorimeter Principle And Application a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. Fundamentals, instrumentation and applications, first edition.. Calorimeter Principle And Application.
From classnotes.org.in
Measurement Of Change In Internal Energy and Enthalpy Chemistry Calorimeter Principle And Application a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Fundamentals, instrumentation and applications, first edition. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Calorimeter Principle And Application.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimeter Principle And Application a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Fundamentals, instrumentation and applications, first edition. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Calorimeter Principle And Application.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Principle And Application Fundamentals, instrumentation and applications, first edition. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. . Calorimeter Principle And Application.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimeter Principle And Application a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Fundamentals, instrumentation and applications, first edition. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Calorimeter Principle And Application.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Principle And Application Fundamentals, instrumentation and applications, first edition. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. a calorimeter. Calorimeter Principle And Application.
From www.youtube.com
CALORIMETRY Its Principle & Applications with Numericals Class 11 Calorimeter Principle And Application calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Fundamentals, instrumentation and applications, first edition. a calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Principle And Application.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter Principle And Application Fundamentals, instrumentation and applications, first edition. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Principle And Application.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter Principle And Application a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Fundamentals, instrumentation and applications, first edition. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change.. Calorimeter Principle And Application.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Principle And Application calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Fundamentals, instrumentation and applications, first edition. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in. Calorimeter Principle And Application.
From www.studypool.com
SOLUTION Calorimeter and principle of calorimetry Studypool Calorimeter Principle And Application calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is. Calorimeter Principle And Application.
From www.youtube.com
Differential Scanning Calorimetry DSC Principle Instrumentation Calorimeter Principle And Application Fundamentals, instrumentation and applications, first edition. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. . Calorimeter Principle And Application.
From www.youtube.com
CALORIMETRY Principle of Calorimetry, Calorimeter, it's application Calorimeter Principle And Application calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Fundamentals, instrumentation and applications, first edition. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Calorimeter Principle And Application.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Principle And Application calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Fundamentals, instrumentation and applications, first edition. a calorimeter is a device used to measure the amount of heat involved in a. Calorimeter Principle And Application.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Principle And Application a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Fundamentals, instrumentation and applications,. Calorimeter Principle And Application.
From www.researchgate.net
Adiabatic calorimeter (a) schematic layout of calorimeter, ice Calorimeter Principle And Application When two bodies of different temperatures (preferably a solid and a liquid) are placed in. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Fundamentals, instrumentation and applications, first edition.. Calorimeter Principle And Application.
From www.researchgate.net
Schematic principle of a metallic calorimeter consisting of an Calorimeter Principle And Application When two bodies of different temperatures (preferably a solid and a liquid) are placed in. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Fundamentals, instrumentation and applications, first edition. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter. Calorimeter Principle And Application.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Principle And Application a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. Fundamentals, instrumentation and applications, first edition. a. Calorimeter Principle And Application.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimeter Principle And Application a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. Fundamentals, instrumentation and applications, first edition. a. Calorimeter Principle And Application.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Principle And Application When two bodies of different temperatures (preferably a solid and a liquid) are placed in. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Fundamentals, instrumentation and applications, first edition. . Calorimeter Principle And Application.
From www.zealinstruments.com
Isoperibol Bomb Calorimeter Principle and Applications zeal Calorimeter Principle And Application a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Fundamentals, instrumentation and applications, first edition. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction. Calorimeter Principle And Application.
From www.zealinstruments.com
Isoperibol Bomb Calorimeter Principle and Applications zeal Calorimeter Principle And Application calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Fundamentals, instrumentation and applications, first edition. calculate. Calorimeter Principle And Application.
From themasterchemistry.com
Calorimeter Types, Principle, Working, Uses Calorimeter Principle And Application a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Fundamentals, instrumentation and applications, first edition. calculate. Calorimeter Principle And Application.
From www.slideshare.net
Differential Scanning Calorimeter Instrumentation.(DSC) Calorimeter Principle And Application a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Fundamentals, instrumentation and applications, first edition. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. calculate heat,. Calorimeter Principle And Application.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Principle And Application a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Fundamentals, instrumentation and applications, first edition. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. When two bodies of different temperatures (preferably a solid and a liquid) are placed in.. Calorimeter Principle And Application.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Principle And Application a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Fundamentals, instrumentation and applications, first edition. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction. Calorimeter Principle And Application.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Principle And Application Fundamentals, instrumentation and applications, first edition. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Calorimeter Principle And Application.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Principle And Application a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. When two bodies of different temperatures (preferably a solid and a liquid) are placed in. calorimeter, device for measuring the. Calorimeter Principle And Application.