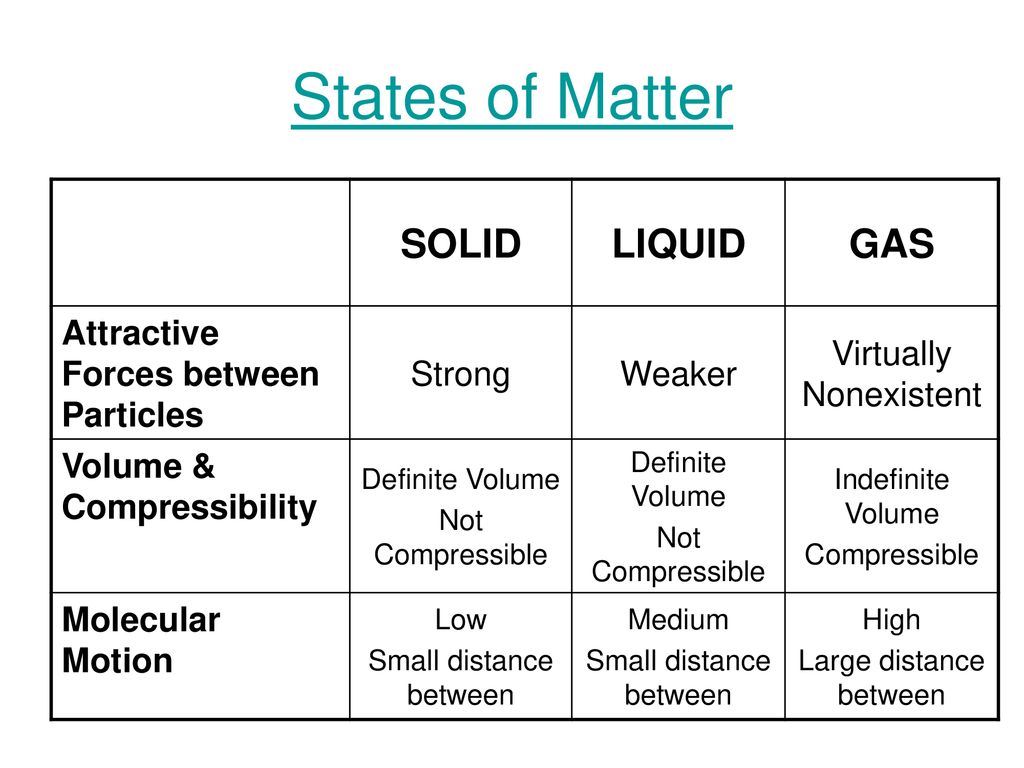
Compressibility Of Solid Liquid And Gas . how are shape and volume used to classify solids, liquids, and gases? this model explains the higher density, greater order, and lower compressibility of liquids versus gases; This is because the particles in the gas are far apart and there are large. unlike solids and liquids, gases are highly compressible. the kinetic molecular theory allows us to explain the existence of the three phases of matter: gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. Why does a gas have no definite shape and no definite.
from slideplayer.com
Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. the kinetic molecular theory allows us to explain the existence of the three phases of matter: unlike solids and liquids, gases are highly compressible. This is because the particles in the gas are far apart and there are large. how are shape and volume used to classify solids, liquids, and gases? compressibility is the measure of how much a given volume of matter decreases when placed under pressure. Why does a gas have no definite shape and no definite. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think.
Unit 7 States of matter and the Behavior of Gases ppt download
Compressibility Of Solid Liquid And Gas This is because the particles in the gas are far apart and there are large. Why does a gas have no definite shape and no definite. how are shape and volume used to classify solids, liquids, and gases? gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. unlike solids and liquids, gases are highly compressible. This is because the particles in the gas are far apart and there are large. the kinetic molecular theory allows us to explain the existence of the three phases of matter: compressibility is the measure of how much a given volume of matter decreases when placed under pressure. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Compressibility Of Solid Liquid And Gas unlike solids and liquids, gases are highly compressible. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. This is because the particles in the gas are far apart and there are. Compressibility Of Solid Liquid And Gas.
From www.pw.live
Class 9 chemistry Notes Matter is our surrounding STATES OF MATTER Compressibility Of Solid Liquid And Gas how are shape and volume used to classify solids, liquids, and gases? gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. This is because the particles in the gas are far apart and there are large. Why does a gas have no definite shape and no definite. compressibility is the measure. Compressibility Of Solid Liquid And Gas.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; compressibility is the measure of how much a given volume of matter decreases when placed under pressure. the kinetic molecular theory allows us to explain the existence of the three phases of matter: gases are very compressible, so when subjected to. Compressibility Of Solid Liquid And Gas.
From slideplayer.com
Chemistry Notes Phases of Matter ppt download Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; compressibility is the measure of how much a given volume of matter decreases when placed under pressure. This is because the particles in the gas are far apart and there are large. Why does a gas have no definite shape and no definite.. Compressibility Of Solid Liquid And Gas.
From www.slideserve.com
PPT Matter & Energy PowerPoint Presentation, free download ID5368269 Compressibility Of Solid Liquid And Gas Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; unlike solids and liquids, gases are highly compressible. compressibility is the measure of how much a given volume of matter decreases when placed. Compressibility Of Solid Liquid And Gas.
From www.slideserve.com
PPT CHAPTER 10 STATES OF MATTER PowerPoint Presentation, free Compressibility Of Solid Liquid And Gas how are shape and volume used to classify solids, liquids, and gases? compressibility is the measure of how much a given volume of matter decreases when placed under pressure. Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. gases are very compressible, so when subjected to high. Compressibility Of Solid Liquid And Gas.
From mungfali.com
Solids Liquids Gases Chart Compressibility Of Solid Liquid And Gas This is because the particles in the gas are far apart and there are large. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; unlike solids and liquids, gases are highly compressible. Why does a gas have no definite shape and no definite. gases are very compressible, so when subjected to. Compressibility Of Solid Liquid And Gas.
From www.slideserve.com
PPT Chapter 14 Properties of Gases PowerPoint Presentation, free Compressibility Of Solid Liquid And Gas how are shape and volume used to classify solids, liquids, and gases? unlike solids and liquids, gases are highly compressible. This is because the particles in the gas are far apart and there are large. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. the kinetic molecular theory allows us. Compressibility Of Solid Liquid And Gas.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Compressibility Of Solid Liquid And Gas Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. Why does a gas have no definite shape and no definite. how are shape and volume used to classify solids, liquids, and gases? unlike solids and liquids, gases are highly compressible. gases are very compressible, so when subjected. Compressibility Of Solid Liquid And Gas.
From www.slideshare.net
Compressibility of solids , liquids and gases Compressibility Of Solid Liquid And Gas Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. Why does a gas have no definite shape and. Compressibility Of Solid Liquid And Gas.
From slideplayer.com
Liquids and Solids Gas Solid low density high compressibility ppt Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; Why does a gas have no definite shape and no definite. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. unlike solids and liquids, gases are highly compressible. how are shape and volume. Compressibility Of Solid Liquid And Gas.
From www.slideserve.com
PPT Chapter 7 States of Matter (gases, liquids and solids) PowerPoint Compressibility Of Solid Liquid And Gas compressibility is the measure of how much a given volume of matter decreases when placed under pressure. unlike solids and liquids, gases are highly compressible. how are shape and volume used to classify solids, liquids, and gases? the kinetic molecular theory allows us to explain the existence of the three phases of matter: this model. Compressibility Of Solid Liquid And Gas.
From www.slideserve.com
PPT Matter PowerPoint Presentation, free download ID8890944 Compressibility Of Solid Liquid And Gas gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; the kinetic molecular theory allows us to explain the existence of the three phases of matter: compressibility is the measure of how much a given volume. Compressibility Of Solid Liquid And Gas.
From exocprdpx.blob.core.windows.net
Solid Liquid And Gas Particles at Mui Jefferson blog Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. unlike solids and liquids, gases are highly compressible. Why does a gas have no definite shape and no definite. compressibility is the measure of how much. Compressibility Of Solid Liquid And Gas.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free Compressibility Of Solid Liquid And Gas unlike solids and liquids, gases are highly compressible. Why does a gas have no definite shape and no definite. the kinetic molecular theory allows us to explain the existence of the three phases of matter: compressibility is the measure of how much a given volume of matter decreases when placed under pressure. this model explains the. Compressibility Of Solid Liquid And Gas.
From dianaschemistrynotes.weebly.com
Chapter 2 CHEMISTRY NOTES Compressibility Of Solid Liquid And Gas compressibility is the measure of how much a given volume of matter decreases when placed under pressure. Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. unlike solids and liquids, gases are highly compressible. gases are very compressible, so when subjected to high pressures, their volumes decrease. Compressibility Of Solid Liquid And Gas.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; how are shape and volume used to classify solids, liquids, and gases? gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. unlike solids and liquids, gases are highly compressible. the kinetic molecular theory allows. Compressibility Of Solid Liquid And Gas.
From www.youtube.com
Volume, compressibility and viscosity of phases of matter YouTube Compressibility Of Solid Liquid And Gas compressibility is the measure of how much a given volume of matter decreases when placed under pressure. Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. Why does a gas have no definite shape and no definite. This is because the particles in the gas are far apart and. Compressibility Of Solid Liquid And Gas.
From www.slideserve.com
PPT The Behavior of Gases PowerPoint Presentation, free download ID Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; Why does a gas have no definite shape and no definite. This is because the particles in the gas are far apart and there are large. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. unlike. Compressibility Of Solid Liquid And Gas.
From coffeytrust.blogspot.com
Compressibility Definition in Fluid Mechanics Coffeytrust Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; how are shape and volume used to classify solids, liquids, and gases? unlike solids and liquids, gases are highly compressible. This is because the particles in the gas are far apart and there are large. the kinetic molecular theory allows us. Compressibility Of Solid Liquid And Gas.
From www.numerade.com
SOLVED Compare the compressibility of solids and liquids. Support your Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; Why does a gas have no definite shape and no definite. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. Compressibility is a measure of how much a substance can be reduced in volume by. Compressibility Of Solid Liquid And Gas.
From www.sciencefacts.net
Physics Page 7 of 19 Science Facts Compressibility Of Solid Liquid And Gas unlike solids and liquids, gases are highly compressible. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; Why does a gas have no definite shape and no definite. the kinetic molecular theory allows us to explain the existence of the three phases of matter: compressibility is the measure of how. Compressibility Of Solid Liquid And Gas.
From www.youtube.com
Matter in Our Surroundings Class 9 Science States of Matter YouTube Compressibility Of Solid Liquid And Gas This is because the particles in the gas are far apart and there are large. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. the kinetic molecular theory allows us to explain the existence of the three phases of matter: Compressibility is a measure of how much a substance can. Compressibility Of Solid Liquid And Gas.
From www.youtube.com
compressibility of solids, liquids and gases YouTube Compressibility Of Solid Liquid And Gas gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. unlike solids and liquids, gases are highly compressible.. Compressibility Of Solid Liquid And Gas.
From blog.mensor.com
What Exactly is The Compressibility of Fluids? Compressibility Of Solid Liquid And Gas unlike solids and liquids, gases are highly compressible. the kinetic molecular theory allows us to explain the existence of the three phases of matter: this model explains the higher density, greater order, and lower compressibility of liquids versus gases; how are shape and volume used to classify solids, liquids, and gases? Compressibility is a measure of. Compressibility Of Solid Liquid And Gas.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Compressibility Of Solid Liquid And Gas gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. the kinetic molecular theory allows us to explain the existence of the three phases of matter: this model explains the higher density, greater order,. Compressibility Of Solid Liquid And Gas.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Compressibility Of Solid Liquid And Gas gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. This is because the particles in the gas are far apart and there are large. the kinetic molecular theory allows us to explain the existence of the three phases of matter: unlike solids and liquids, gases are highly compressible. compressibility is. Compressibility Of Solid Liquid And Gas.
From nursehub.com
States of Matter NurseHub Compressibility Of Solid Liquid And Gas gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. unlike solids and liquids, gases are highly compressible. Why does a gas have no definite shape and no definite. This is because the particles in. Compressibility Of Solid Liquid And Gas.
From slideplayer.com
Unit 7 States of matter and the Behavior of Gases ppt download Compressibility Of Solid Liquid And Gas This is because the particles in the gas are far apart and there are large. Compressibility is a measure of how much a substance can be reduced in volume by the application of pressure. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; unlike solids and liquids, gases are highly compressible. . Compressibility Of Solid Liquid And Gas.
From www.youtube.com
Compressibility of solid, liquid and gas YouTube Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; the kinetic molecular theory allows us to explain the existence of the three phases of matter: compressibility is the measure of how much a given volume of matter decreases when placed under pressure. This is because the particles in the gas are. Compressibility Of Solid Liquid And Gas.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Compressibility Of Solid Liquid And Gas This is because the particles in the gas are far apart and there are large. the kinetic molecular theory allows us to explain the existence of the three phases of matter: compressibility is the measure of how much a given volume of matter decreases when placed under pressure. Why does a gas have no definite shape and no. Compressibility Of Solid Liquid And Gas.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Compressibility Of Solid Liquid And Gas gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; Why does a gas have no definite shape and no definite. unlike solids and liquids, gases are highly compressible. Compressibility is a measure of how much a. Compressibility Of Solid Liquid And Gas.
From www.slideshare.net
Unit 1 Notes Compressibility Of Solid Liquid And Gas the kinetic molecular theory allows us to explain the existence of the three phases of matter: unlike solids and liquids, gases are highly compressible. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. how are shape and volume used to classify solids, liquids, and gases? Compressibility is a. Compressibility Of Solid Liquid And Gas.
From mungfali.com
Expansion Of Solids Liquids And Gases Compressibility Of Solid Liquid And Gas this model explains the higher density, greater order, and lower compressibility of liquids versus gases; the kinetic molecular theory allows us to explain the existence of the three phases of matter: This is because the particles in the gas are far apart and there are large. gases are very compressible, so when subjected to high pressures, their. Compressibility Of Solid Liquid And Gas.
From slidetodoc.com
STATES of MATTER Compare solids liquids and gases Compressibility Of Solid Liquid And Gas compressibility is the measure of how much a given volume of matter decreases when placed under pressure. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; how are shape and volume used to classify solids,. Compressibility Of Solid Liquid And Gas.