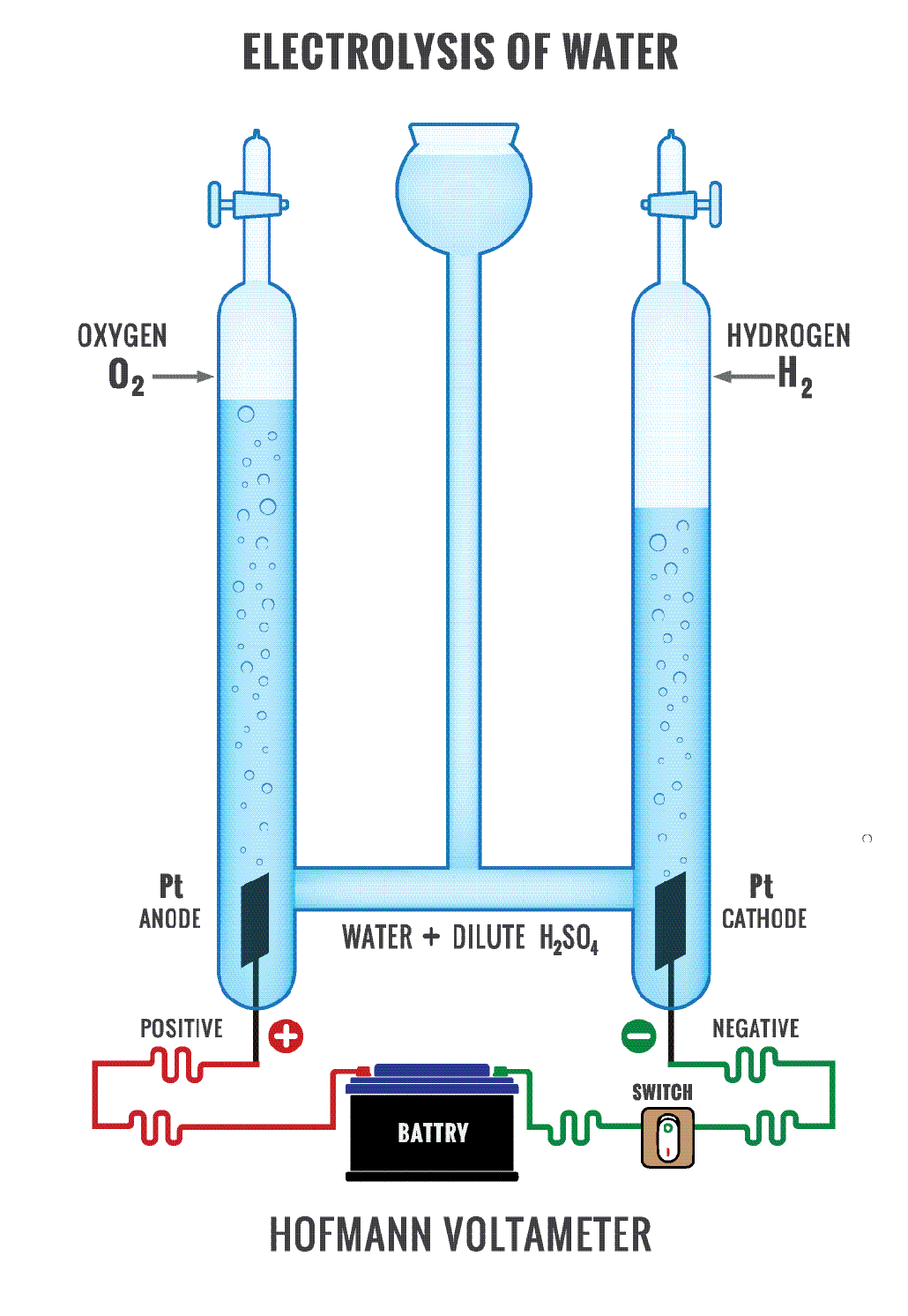
Electrodes Used In Electrolysis Of Water . this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. Electrolysis can also be used to produce h 2 and o 2 from water. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. The electrolytic cell consists of a pair of platinum electrodes. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. the electrolysis of water produces hydrogen and oxygen gases. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. • will be able to explain how hydrogen can be extracted from water.
from www.sciencenewsforstudents.org
the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. the electrolysis of water produces hydrogen and oxygen gases. this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. • will be able to explain how hydrogen can be extracted from water. Electrolysis can also be used to produce h 2 and o 2 from water. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. The electrolytic cell consists of a pair of platinum electrodes. important considerations when preparing electrochemical cells and electrodes for water electrolysis are.
Explainer What is an electrode? Science News for Students
Electrodes Used In Electrolysis Of Water the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. the electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. • will be able to explain how hydrogen can be extracted from water. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. Electrolysis can also be used to produce h 2 and o 2 from water. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction.
From blogs.rsc.org
Electrodes enhance H2 production and sludge in microbial Electrodes Used In Electrolysis Of Water Electrolysis can also be used to produce h 2 and o 2 from water. this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the electrolysis of water produces hydrogen and oxygen gases.. Electrodes Used In Electrolysis Of Water.
From core-global.org
2606 water electrolysis device Hoffman water electrolysis demonstrator Electrodes Used In Electrolysis Of Water in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the electrolysis of water produces hydrogen and oxygen gases. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. important considerations when preparing electrochemical cells and electrodes for water electrolysis are.. Electrodes Used In Electrolysis Of Water.
From pubs.acs.org
Accurate Potentials of Hg/HgO Electrodes Practical Parameters for Electrodes Used In Electrolysis Of Water the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. • will be able to explain how hydrogen can be extracted from water. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. this review provides some basic principles of water electrolysis,. Electrodes Used In Electrolysis Of Water.
From www.researchgate.net
Ni electrode degradation by reversecurrent flow after shutdown of the Electrodes Used In Electrolysis Of Water this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. the electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes. • will be able. Electrodes Used In Electrolysis Of Water.
From www.wikihow.com
How to Electrolyse Water 12 Steps (with Pictures) wikiHow Electrodes Used In Electrolysis Of Water important considerations when preparing electrochemical cells and electrodes for water electrolysis are. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. this review provides. Electrodes Used In Electrolysis Of Water.
From www.res.titech.ac.jp
Development of high performance and durable membraneelectrodeassembly Electrodes Used In Electrolysis Of Water important considerations when preparing electrochemical cells and electrodes for water electrolysis are. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts.. Electrodes Used In Electrolysis Of Water.
From www.dreamstime.com
Electrolysis of Water Splitting H2O Molecules Stock Illustration Electrodes Used In Electrolysis Of Water • will be able to explain how hydrogen can be extracted from water. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. Electrolysis can also be used to produce h 2 and o 2 from water. the electrolysis of water produces hydrogen and oxygen. Electrodes Used In Electrolysis Of Water.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Electrodes Used In Electrolysis Of Water this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. the electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes. Electrolysis can also be used to produce h 2 and o 2 from water. in electrolysis,. Electrodes Used In Electrolysis Of Water.
From hinahanap6dschematic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrodes Used In Electrolysis Of Water the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. Electrolysis can also be used to produce h 2 and o 2 from water. in electrolysis,. Electrodes Used In Electrolysis Of Water.
From childhealthpolicy.vumc.org
😝 Electrolysis using copper electrodes. 0620 QR Dynamic Papers Electrodes Used In Electrolysis Of Water important considerations when preparing electrochemical cells and electrodes for water electrolysis are. Electrolysis can also be used to produce h 2 and o 2 from water. • will be able to explain how hydrogen can be extracted from water. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. electrolysis of water involves passing an. Electrodes Used In Electrolysis Of Water.
From www.alamy.com
Electrolysis hires stock photography and images Alamy Electrodes Used In Electrolysis Of Water the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. the electrolysis of water produces hydrogen and oxygen gases. Electrolysis can also be used to produce h 2 and o 2 from water. important considerations when preparing electrochemical cells and electrodes for water electrolysis. Electrodes Used In Electrolysis Of Water.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Electrodes Used In Electrolysis Of Water this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. Electrolysis can also be used to produce h 2 and o 2 from water. The electrolytic cell consists of a pair of platinum electrodes.. Electrodes Used In Electrolysis Of Water.
From news.europawire.eu
First installation of De Nora’s latest generation of advanced electrode Electrodes Used In Electrolysis Of Water Electrolysis can also be used to produce h 2 and o 2 from water. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. The. Electrodes Used In Electrolysis Of Water.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Electrodes Used In Electrolysis Of Water • will be able to explain how hydrogen can be extracted from water. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. the following video shows the electrolysis of water taking place, using sulfuric acid as. Electrodes Used In Electrolysis Of Water.
From saylordotorg.github.io
Electrochemistry Electrodes Used In Electrolysis Of Water important considerations when preparing electrochemical cells and electrodes for water electrolysis are. the electrolysis of water produces hydrogen and oxygen gases. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. • will be able to explain how hydrogen can be extracted from water. Electrolysis can also be used. Electrodes Used In Electrolysis Of Water.
From energy.gov
Hydrogen Production Electrolysis Department of Energy Electrodes Used In Electrolysis Of Water in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to produce h 2 and o 2 from water. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. The electrolytic cell consists of a pair of platinum electrodes. this review provides. Electrodes Used In Electrolysis Of Water.
From www.alamy.com
Electrolysis experiment hires stock photography and images Alamy Electrodes Used In Electrolysis Of Water the electrolysis of water produces hydrogen and oxygen gases. • will be able to explain how hydrogen can be extracted from water. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. . Electrodes Used In Electrolysis Of Water.
From semcouniversity.com
How the three electrode system works Semco University Semco Electrodes Used In Electrolysis Of Water in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. Electrolysis can also be used to produce h 2 and o 2 from water. The electrolytic cell consists of a pair of platinum electrodes.. Electrodes Used In Electrolysis Of Water.
From mungfali.com
Reference Electrode Diagram Electrodes Used In Electrolysis Of Water in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. Electrolysis can also be used to produce h 2 and o 2 from water. the electrolysis of water produces hydrogen and oxygen. Electrodes Used In Electrolysis Of Water.
From forestparkgolfcourse.com
Water Electrolysis Principle of Water Electrolysis, Important Factors Electrodes Used In Electrolysis Of Water this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. Electrolysis can also be used to produce h 2 and o 2 from water. the electrolysis of. Electrodes Used In Electrolysis Of Water.
From mungfali.com
Glass Electrode Diagram Electrodes Used In Electrolysis Of Water The electrolytic cell consists of a pair of platinum electrodes. • will be able to explain how hydrogen can be extracted from water. the electrolysis of water produces hydrogen and oxygen gases. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. . Electrodes Used In Electrolysis Of Water.
From manualpartwedlock88.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrodes Used In Electrolysis Of Water important considerations when preparing electrochemical cells and electrodes for water electrolysis are. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. Electrolysis can also be used to produce h 2 and o 2 from water. this review provides some basic principles of water electrolysis, key aspects of oer,. Electrodes Used In Electrolysis Of Water.
From www2.mdpi.com
Processes Free FullText CFD Modeling and Experimental Validation Electrodes Used In Electrolysis Of Water • will be able to explain how hydrogen can be extracted from water. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. Electrolysis can also be used to produce h 2 and o 2 from water. The electrolytic cell consists of a pair of platinum. Electrodes Used In Electrolysis Of Water.
From www.sciencephoto.com
Electrolysis of water Stock Image A500/0247 Science Photo Library Electrodes Used In Electrolysis Of Water Electrolysis can also be used to produce h 2 and o 2 from water. • will be able to explain how hydrogen can be extracted from water. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. The electrolytic cell consists of a pair of platinum. Electrodes Used In Electrolysis Of Water.
From phys.org
A powerful catalyst for electrolysis of water that could help harness Electrodes Used In Electrolysis Of Water in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge.. Electrodes Used In Electrolysis Of Water.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation Electrodes Used In Electrolysis Of Water the electrolysis of water produces hydrogen and oxygen gases. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. The electrolytic cell consists of a pair of platinum electrodes. Electrolysis can also be used to produce h 2 and o 2 from water. this review provides some basic principles of water electrolysis, key aspects of. Electrodes Used In Electrolysis Of Water.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Electrodes Used In Electrolysis Of Water The electrolytic cell consists of a pair of platinum electrodes. • will be able to explain how hydrogen can be extracted from water. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. electrolysis of water involves passing an electric current through the water, which. Electrodes Used In Electrolysis Of Water.
From www.myxxgirl.com
Catalyst Based Water Electrolysis Electrochemical Reactions Stock My Electrodes Used In Electrolysis Of Water in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to produce h 2 and o 2 from water. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. The electrolytic cell consists of a pair of platinum electrodes. electrolysis of water involves passing an electric current through. Electrodes Used In Electrolysis Of Water.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Electrodes Used In Electrolysis Of Water the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. • will be able to explain how hydrogen can be extracted from water. Electrolysis can also be used to produce h 2 and o 2 from water. the electrolysis of water produces hydrogen and oxygen. Electrodes Used In Electrolysis Of Water.
From thechemistrynotes.com
Electrolysis of Water Definition, Principle, and Applications Electrodes Used In Electrolysis Of Water in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. the electrolysis of water produces hydrogen and oxygen gases. The. Electrodes Used In Electrolysis Of Water.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Electrodes Used In Electrolysis Of Water • will be able to explain how hydrogen can be extracted from water. Electrolysis can also be used to produce h 2 and o 2 from water. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. the electrolysis of water produces hydrogen and oxygen gases. important considerations when. Electrodes Used In Electrolysis Of Water.
From www.cell.com
practices and benchmarking of foam electrodes in water Electrodes Used In Electrolysis Of Water The electrolytic cell consists of a pair of platinum electrodes. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. • will be able to explain how. Electrodes Used In Electrolysis Of Water.
From www.researchgate.net
Configurations for water electrolysis (a) proton exchange membrane Electrodes Used In Electrolysis Of Water the electrolysis of water produces hydrogen and oxygen gases. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the following video shows the electrolysis of water taking place, using sulfuric acid as a bridge to allow for the transfer of charge. this review provides some basic principles of water electrolysis, key aspects of. Electrodes Used In Electrolysis Of Water.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.58C Describe Experiments to Investigate Electrodes Used In Electrolysis Of Water this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. electrolysis of water involves passing an electric current through the water, which results in its decomposition to hydrogen. important considerations when preparing electrochemical cells and electrodes for water electrolysis are. in electrolysis, an external. Electrodes Used In Electrolysis Of Water.
From pubs.rsc.org
Principles and implementations of electrolysis systems for water Electrodes Used In Electrolysis Of Water • will be able to explain how hydrogen can be extracted from water. the electrolysis of water produces hydrogen and oxygen gases. this review provides some basic principles of water electrolysis, key aspects of oer, and significant criteria for the development of the catalysts. The electrolytic cell consists of a pair of platinum electrodes. electrolysis of water. Electrodes Used In Electrolysis Of Water.