Calcium And Magnesium In Water Analysis . astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. an obvious (if somewhat simplistic) application of the determination of calcium and magnesium in water is testing for. The ed a initially complexes the calcium and. determination of calcium hardness in water by automatic titration. the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. water hardness is a measure of the dissolved calcium and magnesium salts in water and is commonly expressed in. N of calcium andmagnesium in waterdescriptionthe determination of the. determination of calcium and magnesium in a given water sample (edta titrimetric method) written by chief editor wed, 08 jul 20 pm. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. , magnesium, orion 9720bnwp, orion star. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. the magnesium in hard water may provide a moderate health benefit, but calcium can cause serious problems in. edta method for the determination of calcium and magnesium is being used extensively in the analysis of commercial. after adjustment for magnesium levels in drinking water, there was no difference between the groups. both magnesium and calcium can be easily determined by edta titration in the ph 10 against eriochrome black t.
from www.numerade.com
the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. determination of calcium hardness in water by automatic titration. high drinking water calcium and magnesium was associated with lower risk of ischemic and hemorrhagic stroke. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. the magnesium in hard water may provide a moderate health benefit, but calcium can cause serious problems in. the role of calcium and magnesium in plant growth and cell wall deposition cannot be overlooked. while both metals are nutrients that are needed for good human health, high concentrations of these cations (ca2+ and. an obvious (if somewhat simplistic) application of the determination of calcium and magnesium in water is testing for. edta method for the determination of calcium and magnesium is being used extensively in the analysis of commercial. determination of calcium and magnesium in a given water sample (edta titrimetric method) written by chief editor wed, 08 jul 20 pm.
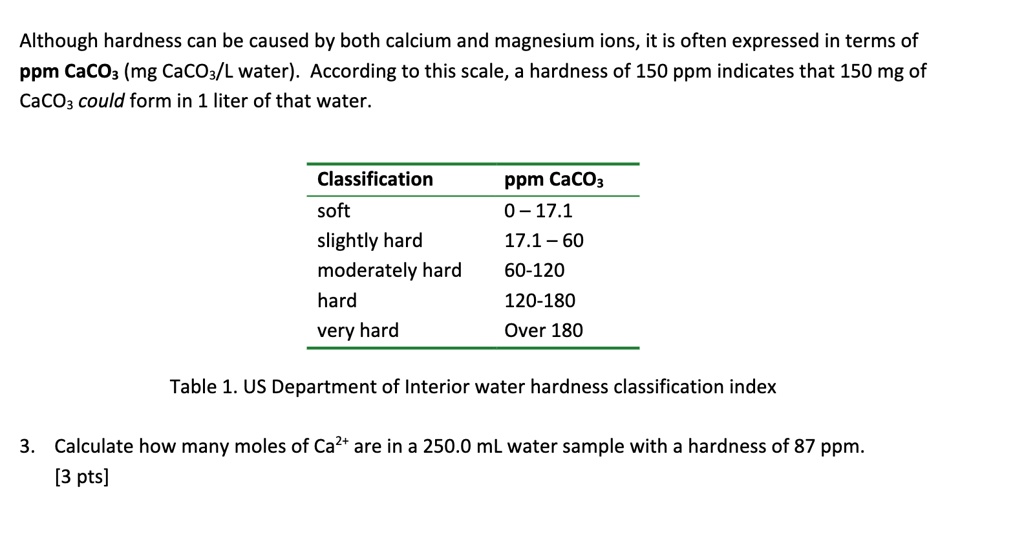
SOLVED Although hardness can be caused by both calcium and magnesium
Calcium And Magnesium In Water Analysis after adjustment for magnesium levels in drinking water, there was no difference between the groups. the role of calcium and magnesium in plant growth and cell wall deposition cannot be overlooked. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. after adjustment for magnesium levels in drinking water, there was no difference between the groups. an obvious (if somewhat simplistic) application of the determination of calcium and magnesium in water is testing for. the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. while both metals are nutrients that are needed for good human health, high concentrations of these cations (ca2+ and. astm d 6919 standard test method for determination of dissolved alkali and alkaline earth cations and ammonium in water and. The ed a initially complexes the calcium and. high drinking water calcium and magnesium was associated with lower risk of ischemic and hemorrhagic stroke. , magnesium, orion 9720bnwp, orion star. the magnesium in hard water may provide a moderate health benefit, but calcium can cause serious problems in. both magnesium and calcium can be easily determined by edta titration in the ph 10 against eriochrome black t. astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. edta method for the determination of calcium and magnesium is being used extensively in the analysis of commercial. If the sample solution initially.
From hydrothailand.com
CALMAG CALCIUM MAGNESIUM HYDRO THAILAND Calcium And Magnesium In Water Analysis the role of calcium and magnesium in plant growth and cell wall deposition cannot be overlooked. Summary of test method from ph 12 to 13 for the determination of calcium alone. the present study evaluated the literature investigating the potential differences in the quantitative calcium. an obvious (if somewhat simplistic) application of the determination of calcium and. Calcium And Magnesium In Water Analysis.
From www.youtube.com
Calcium and magnesium in water UPV YouTube Calcium And Magnesium In Water Analysis water hardness is a measure of the dissolved calcium and magnesium salts in water and is commonly expressed in. high drinking water calcium and magnesium was associated with lower risk of ischemic and hemorrhagic stroke. after adjustment for magnesium levels in drinking water, there was no difference between the groups. an obvious (if somewhat simplistic) application. Calcium And Magnesium In Water Analysis.
From www.youtube.com
Calcium and Magnesium in Water (phenolphthalein added) YouTube Calcium And Magnesium In Water Analysis The ed a initially complexes the calcium and. Summary of test method from ph 12 to 13 for the determination of calcium alone. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. high drinking water calcium and magnesium was associated with lower risk of ischemic and hemorrhagic stroke. after. Calcium And Magnesium In Water Analysis.
From www.youtube.com
How to Perform the Determination of Ca and Mg in Milk Samples and Calcium And Magnesium In Water Analysis the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. determination of calcium and magnesium in a given water sample (edta titrimetric method) written by chief editor wed, 08 jul 20 pm. N of calcium andmagnesium in waterdescriptionthe determination of the. If the sample solution initially. 1.1 these test methods. Calcium And Magnesium In Water Analysis.
From styluspub.presswarehouse.com
Calcium and Magnesium in Drinking Water Calcium And Magnesium In Water Analysis the role of calcium and magnesium in plant growth and cell wall deposition cannot be overlooked. astm d 6919 standard test method for determination of dissolved alkali and alkaline earth cations and ammonium in water and. determination of calcium and magnesium in a given water sample (edta titrimetric method) written by chief editor wed, 08 jul 20. Calcium And Magnesium In Water Analysis.
From labtestsplus.com
Calcium + Magnesium Profile Lab Tests Plus Calcium And Magnesium In Water Analysis the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. the magnesium in hard water may provide a moderate health benefit, but calcium can cause serious problems in. while both metals are nutrients that are needed for good human health, high concentrations of these cations (ca2+ and. astm d8192. Calcium And Magnesium In Water Analysis.
From www.quora.com
Why do magnesium and calcium not react with water, while sodium and Calcium And Magnesium In Water Analysis determination of calcium hardness in water by automatic titration. astm d 6919 standard test method for determination of dissolved alkali and alkaline earth cations and ammonium in water and. , magnesium, orion 9720bnwp, orion star. Summary of test method from ph 12 to 13 for the determination of calcium alone. water hardness is a measure of the. Calcium And Magnesium In Water Analysis.
From householdmag.com
How to Remove Calcium & Magnesium From Water HouseHoldMag Calcium And Magnesium In Water Analysis the procedure is intended for the analysis of raw and drinking waters and can be used for waters having a calcium content of up to. astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water with an optical sensor. the present study evaluated the literature investigating the potential differences in the quantitative. Calcium And Magnesium In Water Analysis.
From www.youtube.com
Calcium and Magnesium ion concentration determination with EDTA Calcium And Magnesium In Water Analysis astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. both magnesium and calcium can be easily determined by edta titration in the ph 10 against eriochrome black t. the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. , magnesium,. Calcium And Magnesium In Water Analysis.
From constructionhow.com
Determination of CalciumMagnesium Hardness of Water Construction How Calcium And Magnesium In Water Analysis high drinking water calcium and magnesium was associated with lower risk of ischemic and hemorrhagic stroke. edta method for the determination of calcium and magnesium is being used extensively in the analysis of commercial. astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water with an optical sensor. Summary of test method. Calcium And Magnesium In Water Analysis.
From exoypnorc.blob.core.windows.net
Metal In Water Reaction at Glenn King blog Calcium And Magnesium In Water Analysis astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water with an optical sensor. Summary of test method from ph 12 to 13 for the determination of calcium alone. The ed a initially complexes the calcium and. edta method for the determination of calcium and magnesium is being used extensively in the analysis. Calcium And Magnesium In Water Analysis.
From www.youtube.com
Why does Magnesium react with Water? YouTube Calcium And Magnesium In Water Analysis astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water with an optical sensor. the present study evaluated the literature investigating the potential differences in the quantitative calcium. the magnesium in hard water may provide a moderate health benefit, but calcium can cause serious problems in. Summary of test method from ph. Calcium And Magnesium In Water Analysis.
From www.degruyter.com
Synthesis of magnesium carbonate hydrate from natural talc Calcium And Magnesium In Water Analysis astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water with an optical sensor. the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. edta method for the determination of calcium and magnesium is being used extensively in the analysis of commercial. the procedure. Calcium And Magnesium In Water Analysis.
From www.numerade.com
SOLVED Although hardness can be caused by both calcium and magnesium Calcium And Magnesium In Water Analysis both magnesium and calcium can be easily determined by edta titration in the ph 10 against eriochrome black t. astm d 6919 standard test method for determination of dissolved alkali and alkaline earth cations and ammonium in water and. astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water with an optical. Calcium And Magnesium In Water Analysis.
From www.aquacell-waterontharder.nl
Calcium magnesium in drinkwater? AquaCell® waterontharder Calcium And Magnesium In Water Analysis N of calcium andmagnesium in waterdescriptionthe determination of the. the procedure is intended for the analysis of raw and drinking waters and can be used for waters having a calcium content of up to. , magnesium, orion 9720bnwp, orion star. after adjustment for magnesium levels in drinking water, there was no difference between the groups. astm d. Calcium And Magnesium In Water Analysis.
From integrativepro.com
Liquid Calcium Magnesium (21) Integrative Therapeutics® Calcium And Magnesium In Water Analysis while both metals are nutrients that are needed for good human health, high concentrations of these cations (ca2+ and. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. The ed a initially complexes the calcium and. an obvious (if somewhat simplistic) application of the determination of calcium and magnesium. Calcium And Magnesium In Water Analysis.
From slideplayer.com
Chemicals Around us Water Near You Percents and Stuff 5 pt 5 pt 5 pt Calcium And Magnesium In Water Analysis If the sample solution initially. the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. an obvious (if somewhat simplistic) application of the determination of calcium and magnesium in water is testing for. while both metals are nutrients that are needed for good human health, high concentrations of these cations. Calcium And Magnesium In Water Analysis.
From material-properties.org
Magnesium and Calcium Comparison Properties Material Properties Calcium And Magnesium In Water Analysis N of calcium andmagnesium in waterdescriptionthe determination of the. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. edta method for the determination of calcium and magnesium is being used extensively in the analysis of commercial. water hardness is a measure of the dissolved calcium and magnesium salts in. Calcium And Magnesium In Water Analysis.
From mineralwaterprojectinformation.org
What is Packaged Natural Mineral Water Actually ? Mineral Water Calcium And Magnesium In Water Analysis determination of calcium and magnesium in a given water sample (edta titrimetric method) written by chief editor wed, 08 jul 20 pm. determination of calcium hardness in water by automatic titration. astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. both magnesium and calcium can be. Calcium And Magnesium In Water Analysis.
From www.researchgate.net
Water quality results for pH, bicarbonate, calcium, and magnesium Calcium And Magnesium In Water Analysis the role of calcium and magnesium in plant growth and cell wall deposition cannot be overlooked. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. the magnesium in hard water may provide a moderate health benefit, but calcium can cause serious problems in. water hardness is a measure. Calcium And Magnesium In Water Analysis.
From www.researchgate.net
(PDF) Calcium and Magnesium Requirements of Ilex crenata ‘Helleri’ Calcium And Magnesium In Water Analysis 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. , magnesium, orion 9720bnwp, orion star. astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. determination of calcium and magnesium in a given water sample (edta titrimetric method) written by. Calcium And Magnesium In Water Analysis.
From www.studocu.com
EDTA Titration for Determination of calcium and magnesium In this Calcium And Magnesium In Water Analysis 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. an obvious (if somewhat simplistic) application of the determination of calcium and magnesium in water is testing for. the procedure is intended. Calcium And Magnesium In Water Analysis.
From www.youtube.com
How to remove Calcium and Magnesium from water? [each step of the Calcium And Magnesium In Water Analysis astm d 6919 standard test method for determination of dissolved alkali and alkaline earth cations and ammonium in water and. The ed a initially complexes the calcium and. astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. the present study evaluated the literature investigating the potential differences. Calcium And Magnesium In Water Analysis.
From www.youtube.com
Estimation of calcium and magnesium in a given water sample YouTube Calcium And Magnesium In Water Analysis water hardness is a measure of the dissolved calcium and magnesium salts in water and is commonly expressed in. astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water with an optical sensor. after adjustment for magnesium levels in drinking water, there was no difference between the groups. 1.1 these test. Calcium And Magnesium In Water Analysis.
From www.researchgate.net
Calcium and Magnesium Cycle. Download Scientific Diagram Calcium And Magnesium In Water Analysis the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. determination of calcium hardness in water by automatic titration. an obvious (if somewhat simplistic) application of the determination of calcium and magnesium in water is testing for. N of calcium andmagnesium in waterdescriptionthe determination of the. astm d8192 describes. Calcium And Magnesium In Water Analysis.
From www.ukaps.org
Water report, do I have enough calcium and magnesium? UK Aquatic Calcium And Magnesium In Water Analysis astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water with an optical sensor. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. water hardness is a measure of the dissolved calcium and magnesium salts in water and is commonly expressed in. after. Calcium And Magnesium In Water Analysis.
From www.researchgate.net
Effect of calcium and magnesium (A) and NOM (B) on biocatalytic Calcium And Magnesium In Water Analysis , magnesium, orion 9720bnwp, orion star. the present study evaluated the literature investigating the potential differences in the quantitative calcium. the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. determination of. Calcium And Magnesium In Water Analysis.
From www.researchgate.net
Effect of calcium and magnesium in the drinking water on the incidence Calcium And Magnesium In Water Analysis an obvious (if somewhat simplistic) application of the determination of calcium and magnesium in water is testing for. 1.1 these test methods cover the determination of calcium and magnesium in water by complexometric titration and. edta method for the determination of calcium and magnesium is being used extensively in the analysis of commercial. the simple definition. Calcium And Magnesium In Water Analysis.
From brainreference.com
Liquid Calcium with Magnesium Review 8 Reasons To Consider Calcium And Magnesium In Water Analysis astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. water hardness is a measure of the dissolved calcium and magnesium salts in water and is commonly expressed in. the present study evaluated the literature investigating the potential differences in the quantitative calcium. N of calcium andmagnesium in. Calcium And Magnesium In Water Analysis.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free Calcium And Magnesium In Water Analysis astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. Summary of test method from ph 12 to 13 for the determination of calcium alone. the procedure is intended for the analysis of raw and drinking waters and can be used for waters having a calcium content of up. Calcium And Magnesium In Water Analysis.
From studylib.net
Determination of Total Calcium and Magnesium Calcium And Magnesium In Water Analysis water hardness is a measure of the dissolved calcium and magnesium salts in water and is commonly expressed in. the procedure is intended for the analysis of raw and drinking waters and can be used for waters having a calcium content of up to. determination of calcium and magnesium in a given water sample (edta titrimetric method). Calcium And Magnesium In Water Analysis.
From www.researchgate.net
Raman spectra of calcium and magnesium carbonates dolomite (UNIMIB Calcium And Magnesium In Water Analysis astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. astm d 6919 standard test method for determination of dissolved alkali and alkaline earth cations and ammonium in water and. the present study evaluated the literature investigating the potential differences in the quantitative calcium. an obvious (if. Calcium And Magnesium In Water Analysis.
From www.desertcart.com.eg
Monitor Calcium/Magnesium Low Salinity (05 PPT) Test KIT (120 Tests Calcium And Magnesium In Water Analysis astm d8192 describes the photometric titration of the total, calcium, and magnesium hardness in water l sensor for objective. after adjustment for magnesium levels in drinking water, there was no difference between the groups. the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. while both metals are nutrients. Calcium And Magnesium In Water Analysis.
From www.researchgate.net
Calcium and magnesium contents extracted with different extraction Calcium And Magnesium In Water Analysis water hardness is a measure of the dissolved calcium and magnesium salts in water and is commonly expressed in. , magnesium, orion 9720bnwp, orion star. The ed a initially complexes the calcium and. astm d 6919 standard test method for determination of dissolved alkali and alkaline earth cations and ammonium in water and. Summary of test method from. Calcium And Magnesium In Water Analysis.
From www.researchgate.net
Calcium and Magnesium hardness(mg/L) values in the study sites Calcium And Magnesium In Water Analysis both magnesium and calcium can be easily determined by edta titration in the ph 10 against eriochrome black t. after adjustment for magnesium levels in drinking water, there was no difference between the groups. determination of calcium hardness in water by automatic titration. N of calcium andmagnesium in waterdescriptionthe determination of the. 1.1 these test methods. Calcium And Magnesium In Water Analysis.