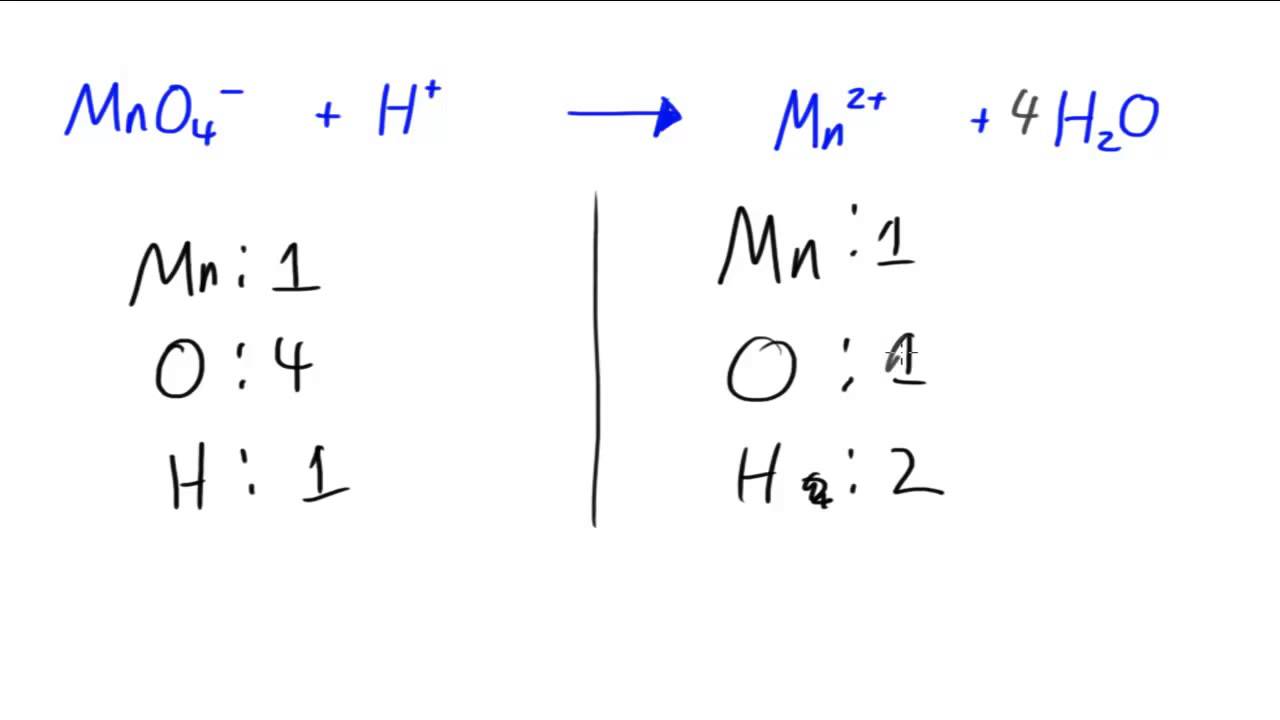
Balance Charge With Electrons . An easy way to remember this is to think of the charges: Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An element's charge is reduced if it gains electrons (an acronym to. It also means that the electrons and charges are balanced: Balance charge by adding electrons. Balance the charge by adding electrons. Balance the charge of each equation with electrons. The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Cr → cr 3 + + 3e − fe.
from www.youtube.com
The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. It also means that the electrons and charges are balanced: Balance the charge by adding electrons. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: Balance the charge of each equation with electrons. An easy way to remember this is to think of the charges: Balance charge by adding electrons. Cr → cr 3 + + 3e − fe. An element's charge is reduced if it gains electrons (an acronym to.
OCR AS Chemistry Balancing Ionic Equations example 2 YouTube
Balance Charge With Electrons Balance charge by adding electrons. Balance the charge of each equation with electrons. Balance charge by adding electrons. The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Cr → cr 3 + + 3e − fe. Balance the charge by adding electrons. It also means that the electrons and charges are balanced: Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An element's charge is reduced if it gains electrons (an acronym to. An easy way to remember this is to think of the charges:
From www.slideserve.com
PPT Electrochemistry Lesson 5 Balancing Half Reactions PowerPoint Balance Charge With Electrons Cr → cr 3 + + 3e − fe. Balance the charge by adding electrons. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. An element's charge is reduced if it gains electrons. Balance Charge With Electrons.
From www.youtube.com
Calculating charge using Electrons and Protons YouTube Balance Charge With Electrons Balance the charge of each equation with electrons. Cr → cr 3 + + 3e − fe. The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. It also means that the electrons and charges are balanced: An easy way to remember this is to think of the charges: Balance charge by adding. Balance Charge With Electrons.
From chemistry101efhs.weebly.com
Balancing Equations Chemistry 101 Balance Charge With Electrons Balance the charge by adding electrons. Balance the charge of each equation with electrons. An easy way to remember this is to think of the charges: Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: The number of electrons lost during oxidation is equal to the number of electrons gained. Balance Charge With Electrons.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii Balance Charge With Electrons Balance the charge of each equation with electrons. An easy way to remember this is to think of the charges: Balance the charge by adding electrons. It also means that the electrons and charges are balanced: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Balance charge by adding electrons. Cr →. Balance Charge With Electrons.
From www.slideserve.com
PPT Balancing Redox Equations following the electrons PowerPoint Balance Charge With Electrons It also means that the electrons and charges are balanced: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Cr → cr 3 + + 3e − fe. An element's charge is reduced if it gains electrons (an acronym to. An easy way to remember this is to think of the charges:. Balance Charge With Electrons.
From slideplayer.com
OXIDATION AND REDUCTION ppt download Balance Charge With Electrons Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An easy way to remember this is to think of the charges: An element's charge is reduced if it gains electrons (an acronym to. Cr → cr 3 + + 3e − fe. It also means that the electrons and charges. Balance Charge With Electrons.
From www.youtube.com
Balancing Chemical Equations YouTube Balance Charge With Electrons Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: Balance the charge of each equation with electrons. Cr → cr 3 + + 3e − fe. Balance the charge by adding electrons. An element's charge is reduced if it gains electrons (an acronym to. Balance charge by adding electrons. The. Balance Charge With Electrons.
From rosstable.blogspot.com
The Ross Periodic Table Core Charge Its Periodicity Across the Table Balance Charge With Electrons An element's charge is reduced if it gains electrons (an acronym to. Balance the charge by adding electrons. Balance charge by adding electrons. Balance the charge of each equation with electrons. Cr → cr 3 + + 3e − fe. An easy way to remember this is to think of the charges: Then we include the appropriate number of electrons. Balance Charge With Electrons.
From www.slideserve.com
PPT Balancing Redox Equations PowerPoint Presentation, free download Balance Charge With Electrons Balance the charge by adding electrons. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. It also means that the electrons and charges are balanced: An easy way to remember this is to. Balance Charge With Electrons.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Balance Charge With Electrons Cr → cr 3 + + 3e − fe. The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Balance the charge by adding electrons. An element's charge is reduced if it gains electrons (an acronym to. It also means that the electrons and charges are balanced: Balance charge by adding electrons. An. Balance Charge With Electrons.
From slideplayer.com
Ion Electron Method Ch ppt download Balance Charge With Electrons An easy way to remember this is to think of the charges: Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Balance the charge of each equation with electrons. Balance the charge by. Balance Charge With Electrons.
From santiagomeowmeyer.blogspot.com
Why Are Only Valence Electrons Involved in Bonding Balance Charge With Electrons Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An element's charge is reduced if it gains electrons (an acronym to. Balance the charge of each equation with electrons. Cr → cr 3 + + 3e − fe. Balance the charge by adding electrons. Balance charge by adding electrons. The. Balance Charge With Electrons.
From www.youtube.com
OCR AS Chemistry Balancing Ionic Equations example 2 YouTube Balance Charge With Electrons Balance the charge by adding electrons. An element's charge is reduced if it gains electrons (an acronym to. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. An easy way to remember this. Balance Charge With Electrons.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? Balance Charge With Electrons Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Balance the charge by adding electrons. Balance charge by adding electrons. It also means that the electrons and charges are balanced: Cr → cr. Balance Charge With Electrons.
From slideplayer.com
Half Reactions. ppt download Balance Charge With Electrons An element's charge is reduced if it gains electrons (an acronym to. The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: It also means that the electrons and charges are balanced: An easy. Balance Charge With Electrons.
From www.pinterest.com
Four covalent bonds. Carbon has four valence electrons and here a Balance Charge With Electrons Balance the charge by adding electrons. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: Balance the charge of each equation with electrons. An easy way to remember this is to think of the charges: Balance charge by adding electrons. An element's charge is reduced if it gains electrons (an. Balance Charge With Electrons.
From www.mindomo.com
Energy Mind Map Balance Charge With Electrons The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Balance the charge by adding electrons. It also means that the electrons and charges are balanced: Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An easy way to remember this is to. Balance Charge With Electrons.
From mungfali.com
Charge Balance Equation Balance Charge With Electrons It also means that the electrons and charges are balanced: An easy way to remember this is to think of the charges: An element's charge is reduced if it gains electrons (an acronym to. Cr → cr 3 + + 3e − fe. Balance the charge of each equation with electrons. Balance the charge by adding electrons. The number of. Balance Charge With Electrons.
From www.slideserve.com
PPT RedOx Chemistry PowerPoint Presentation, free download ID3201551 Balance Charge With Electrons Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: Cr → cr 3 + + 3e − fe. Balance the charge by adding electrons. An easy way to remember this is to think of the charges: It also means that the electrons and charges are balanced: The number of electrons. Balance Charge With Electrons.
From www.slideserve.com
PPT RedOx Chemistry PowerPoint Presentation, free download ID3201551 Balance Charge With Electrons Balance the charge by adding electrons. Balance the charge of each equation with electrons. Cr → cr 3 + + 3e − fe. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An easy way to remember this is to think of the charges: The number of electrons lost during. Balance Charge With Electrons.
From www.animalia-life.club
Periodic Table Of Elements With All Charges Balance Charge With Electrons Balance charge by adding electrons. Cr → cr 3 + + 3e − fe. An easy way to remember this is to think of the charges: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Then we include the appropriate number of electrons on the proper side to balance the charges for. Balance Charge With Electrons.
From slideplayer.com
Electric Current Chapter ppt download Balance Charge With Electrons An easy way to remember this is to think of the charges: An element's charge is reduced if it gains electrons (an acronym to. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: Balance the charge by adding electrons. Balance charge by adding electrons. The number of electrons lost during. Balance Charge With Electrons.
From www.slideserve.com
PPT The Electric Force PowerPoint Presentation, free download ID498523 Balance Charge With Electrons Cr → cr 3 + + 3e − fe. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. It also means that the electrons and charges are balanced: An element's charge is reduced. Balance Charge With Electrons.
From cmc-lubumbashi.com
Balancing redox reactions practice Balance Charge With Electrons An element's charge is reduced if it gains electrons (an acronym to. Balance the charge of each equation with electrons. Balance the charge by adding electrons. The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Cr → cr 3 + + 3e − fe. Then we include the appropriate number of electrons. Balance Charge With Electrons.
From slideplayer.com
Atoms Vocab. ppt download Balance Charge With Electrons Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An element's charge is reduced if it gains electrons (an acronym to. Balance charge by adding electrons. It also means that the electrons and charges are balanced: Balance the charge of each equation with electrons. Cr → cr 3 + +. Balance Charge With Electrons.
From slideplayer.com
Chapter Introduction Lesson 1 Atoms Lesson 2 Elements ppt download Balance Charge With Electrons Cr → cr 3 + + 3e − fe. It also means that the electrons and charges are balanced: Balance charge by adding electrons. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An element's charge is reduced if it gains electrons (an acronym to. The number of electrons lost. Balance Charge With Electrons.
From www.numerade.com
SOLVED 4. Balance charge by adding electrons. 5. Multiply each half Balance Charge With Electrons Balance the charge of each equation with electrons. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Cr → cr 3 + + 3e − fe. It also means that the electrons and. Balance Charge With Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Balance Charge With Electrons Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. An easy way to remember this is to think of the charges: Balance charge by adding electrons. An element's charge is reduced if it. Balance Charge With Electrons.
From users.highland.edu
Lewis Structures and Covalent Bonding Balance Charge With Electrons Balance the charge of each equation with electrons. The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: Balance charge by adding electrons. It also means that the electrons and charges are balanced: An. Balance Charge With Electrons.
From www.numerade.com
SOLVED Label the parts of an atom ATOMIC STRUCTURE Complete the table Balance Charge With Electrons Cr → cr 3 + + 3e − fe. Balance charge by adding electrons. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An element's charge is reduced if it gains electrons (an acronym to. Balance the charge by adding electrons. It also means that the electrons and charges are. Balance Charge With Electrons.
From www.slideserve.com
PPT Balancing Redox Reactions PowerPoint Presentation, free download Balance Charge With Electrons Balance the charge of each equation with electrons. An easy way to remember this is to think of the charges: Balance the charge by adding electrons. Balance charge by adding electrons. Cr → cr 3 + + 3e − fe. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: It. Balance Charge With Electrons.
From www.slideserve.com
PPT Balancing Redox Equations following the electrons PowerPoint Balance Charge With Electrons The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Balance charge by adding electrons. Balance the charge by adding electrons. An element's charge is reduced if it gains electrons (an acronym to. Balance the charge of each equation with electrons. It also means that the electrons and charges are balanced: An easy. Balance Charge With Electrons.
From periodictable.me
What are the Difference Between Charge and Electron? Dynamic Periodic Balance Charge With Electrons Balance charge by adding electrons. Balance the charge of each equation with electrons. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An element's charge is reduced if it gains electrons (an acronym to. Cr → cr 3 + + 3e − fe. An easy way to remember this is. Balance Charge With Electrons.
From periodictable.me
What are the Difference Between Charge and Electron? Dynamic Periodic Balance Charge With Electrons Balance the charge of each equation with electrons. Cr → cr 3 + + 3e − fe. The number of electrons lost during oxidation is equal to the number of electrons gained during reduction. Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: Balance the charge by adding electrons. It. Balance Charge With Electrons.
From studyqueries.com
Charge Of Proton Definition, Mass & More Balance Charge With Electrons Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction: An element's charge is reduced if it gains electrons (an acronym to. Balance charge by adding electrons. Balance the charge by adding electrons. Balance the charge of each equation with electrons. The number of electrons lost during oxidation is equal to. Balance Charge With Electrons.