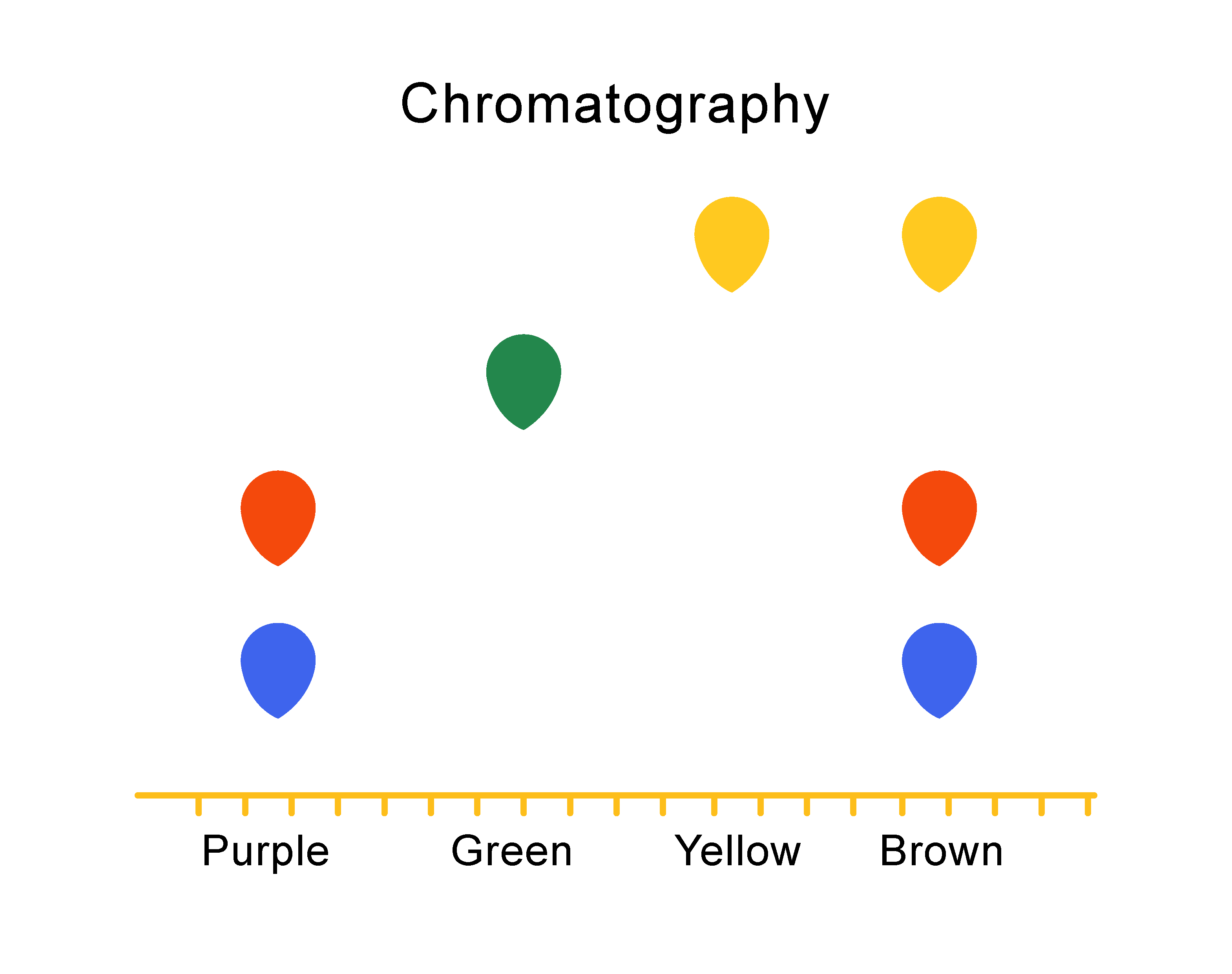
Explain Why The Chromatography Spot Should Be Small . The spots should be very small, around \(2 \: Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. Make sure enough sample is spotted on the plate. The silica gel (or the alumina) is. If the spot is not. It is also known as open column chromatography, drop strip,. No, you should always spot the samples slightly above the level of. A purple spot should be seen. Should you spot tlc samples right at the bottom of the plate? A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. Using a capillary tube, a. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot.
from jetpaper.web.fc2.com
No, you should always spot the samples slightly above the level of. A purple spot should be seen. If the spot is not. The spots should be very small, around \(2 \: The silica gel (or the alumina) is. Using a capillary tube, a. Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. Make sure enough sample is spotted on the plate. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot. It is also known as open column chromatography, drop strip,.
what industries use chromatography and what do they use this technique for?
Explain Why The Chromatography Spot Should Be Small A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot. Make sure enough sample is spotted on the plate. A purple spot should be seen. If the spot is not. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. The silica gel (or the alumina) is. Using a capillary tube, a. The spots should be very small, around \(2 \: A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot. Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. No, you should always spot the samples slightly above the level of. It is also known as open column chromatography, drop strip,. Should you spot tlc samples right at the bottom of the plate?
From revisechemistry.uk
Purity, Formulations, Chromatography and Identification of Common Gases Explain Why The Chromatography Spot Should Be Small Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. It is also known as open column chromatography, drop strip,. The spots should be very small, around \(2 \: A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation.. Explain Why The Chromatography Spot Should Be Small.
From owlcation.com
Thin Layer Chromatography (TLC) Principle and Procedure Owlcation Explain Why The Chromatography Spot Should Be Small A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. The spots should be very small, around \(2 \: A purple spot should be seen. The silica gel (or the alumina) is. Should you spot tlc samples right at the bottom of the plate? Using a capillary tube, a. No, you. Explain Why The Chromatography Spot Should Be Small.
From www.sciencephoto.com
Paper chromatography Stock Image A500/0258 Science Photo Library Explain Why The Chromatography Spot Should Be Small Make sure enough sample is spotted on the plate. It is also known as open column chromatography, drop strip,. Should you spot tlc samples right at the bottom of the plate? The silica gel (or the alumina) is. Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and.. Explain Why The Chromatography Spot Should Be Small.
From keystagewiki.com
Chromatography Key Stage Wiki Explain Why The Chromatography Spot Should Be Small Using a capillary tube, a. The spots should be very small, around \(2 \: Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. Should you spot tlc samples right at the bottom of the plate? A solution of the sample containing a mixture of compounds is applied. Explain Why The Chromatography Spot Should Be Small.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC Explain Why The Chromatography Spot Should Be Small It is also known as open column chromatography, drop strip,. A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. No, you should always spot the samples slightly above the level of. If the spot is not. The spots should be very small, around \(2 \: Using a capillary tube, a.. Explain Why The Chromatography Spot Should Be Small.
From in.pinterest.com
Chemistry Classroom, Teaching Chemistry, Biology Teacher, Cell Biology Explain Why The Chromatography Spot Should Be Small Using a capillary tube, a. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. Make sure enough sample. Explain Why The Chromatography Spot Should Be Small.
From www.science-sparks.com
Paper Chromatography Experiment Explain Why The Chromatography Spot Should Be Small No, you should always spot the samples slightly above the level of. Using a capillary tube, a. Should you spot tlc samples right at the bottom of the plate? A purple spot should be seen. The spots should be very small, around \(2 \: A solution of the sample containing a mixture of compounds is applied to the layer of. Explain Why The Chromatography Spot Should Be Small.
From qva.com.au
😂 Chromatography lab report. Introduction of Paper Chromatography. 2019 Explain Why The Chromatography Spot Should Be Small Using a capillary tube, a. No, you should always spot the samples slightly above the level of. Should you spot tlc samples right at the bottom of the plate? Make sure enough sample is spotted on the plate. The spots should be very small, around \(2 \: The silica gel (or the alumina) is. Deliver a very small spot of. Explain Why The Chromatography Spot Should Be Small.
From www.youtube.com
Column chromatography principle and working YouTube Explain Why The Chromatography Spot Should Be Small A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. No, you should always spot the samples slightly above the level of. Using a capillary tube, a. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot.. Explain Why The Chromatography Spot Should Be Small.
From owlcation.com
What Is Paper Chromatography and How Does It Work? Owlcation Explain Why The Chromatography Spot Should Be Small Using a capillary tube, a. No, you should always spot the samples slightly above the level of. A purple spot should be seen. The silica gel (or the alumina) is. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot. If the spot is not. In. Explain Why The Chromatography Spot Should Be Small.
From www.nagwa.com
Lesson Video Chromatography Nagwa Explain Why The Chromatography Spot Should Be Small A purple spot should be seen. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. Should you spot tlc samples right. Explain Why The Chromatography Spot Should Be Small.
From www.numerade.com
Why do you mark the chromatography paper with a pencil, not a pen? 2 Explain Why The Chromatography Spot Should Be Small Using a capillary tube, a. If the spot is not. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. Make sure enough sample is spotted on the plate. The silica gel (or the alumina) is. It is also known as. Explain Why The Chromatography Spot Should Be Small.
From www.lookfordiagnosis.com
Chromatography, ion exchange Explain Why The Chromatography Spot Should Be Small Should you spot tlc samples right at the bottom of the plate? The silica gel (or the alumina) is. It is also known as open column chromatography, drop strip,. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot. No, you should always spot the samples. Explain Why The Chromatography Spot Should Be Small.
From www.youtube.com
Thin Layer Chromatography Performing an Analysis YouTube Explain Why The Chromatography Spot Should Be Small Using a capillary tube, a. The spots should be very small, around \(2 \: Should you spot tlc samples right at the bottom of the plate? The silica gel (or the alumina) is. No, you should always spot the samples slightly above the level of. Deliver a very small spot of material on the pencil line of the appropriate lane. Explain Why The Chromatography Spot Should Be Small.
From www.chemistrystudent.com
Chromatography (ALevel) ChemistryStudent Explain Why The Chromatography Spot Should Be Small Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. A purple spot should be seen. If the spot is not. A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. No, you should always spot the samples slightly. Explain Why The Chromatography Spot Should Be Small.
From laptrinhx.com
Paper Chromatography Experiment LaptrinhX / News Explain Why The Chromatography Spot Should Be Small A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. Should you spot tlc samples right at the bottom of the plate? Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. The silica gel (or the alumina) is.. Explain Why The Chromatography Spot Should Be Small.
From group.chem.iastate.edu
VanVeller Lab Resources Explain Why The Chromatography Spot Should Be Small Should you spot tlc samples right at the bottom of the plate? Using a capillary tube, a. If the spot is not. Make sure enough sample is spotted on the plate. It is also known as open column chromatography, drop strip,. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a. Explain Why The Chromatography Spot Should Be Small.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC Explain Why The Chromatography Spot Should Be Small Make sure enough sample is spotted on the plate. A purple spot should be seen. Should you spot tlc samples right at the bottom of the plate? If the spot is not. No, you should always spot the samples slightly above the level of. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin. Explain Why The Chromatography Spot Should Be Small.
From www.youtube.com
Paper chromatography/Radial paper chromatography (Principle, procedure Explain Why The Chromatography Spot Should Be Small A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. Make sure enough sample is spotted on the plate. A solution of. Explain Why The Chromatography Spot Should Be Small.
From ar.inspiredpencil.com
Thin Layer Chromatography Set Up Explain Why The Chromatography Spot Should Be Small The spots should be very small, around \(2 \: A purple spot should be seen. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot. If the spot is not. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin. Explain Why The Chromatography Spot Should Be Small.
From mungfali.com
Paper Chromatography Labelled Diagram Explain Why The Chromatography Spot Should Be Small Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one. Explain Why The Chromatography Spot Should Be Small.
From www.youtube.com
Chromatography Organic Chemistry IIT JEE, NEET, CBSE YouTube Explain Why The Chromatography Spot Should Be Small A purple spot should be seen. The spots should be very small, around \(2 \: If the spot is not. A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as. Explain Why The Chromatography Spot Should Be Small.
From www.slideshare.net
Planar Chromatography Explain Why The Chromatography Spot Should Be Small Using a capillary tube, a. If the spot is not. It is also known as open column chromatography, drop strip,. A purple spot should be seen. Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. In thin layer chromatography the solid phase (silica gel or alumina) is. Explain Why The Chromatography Spot Should Be Small.
From aspen-blogorr.blogspot.com
Why Is the Start Line in Chromatography Drawn in Pencil Explain Why The Chromatography Spot Should Be Small Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. The silica gel (or the alumina) is. Make sure enough sample is spotted on the plate. The spots should be very small, around \(2 \: Using a capillary tube, a. A chemist often needs to find the “sweet. Explain Why The Chromatography Spot Should Be Small.
From www.youtube.com
Paper Chromatography Science Project YouTube Explain Why The Chromatography Spot Should Be Small It is also known as open column chromatography, drop strip,. No, you should always spot the samples slightly above the level of. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. The silica gel (or the alumina) is. A solution. Explain Why The Chromatography Spot Should Be Small.
From www.vecteezy.com
Paper chromatography analytical method for the separation of a mixture Explain Why The Chromatography Spot Should Be Small In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. The silica gel (or the alumina) is. A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. No, you should always spot. Explain Why The Chromatography Spot Should Be Small.
From www.chegg.com
Solved Briefly explain why the chromatography column should Explain Why The Chromatography Spot Should Be Small In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. It is also known as open column chromatography, drop strip,. If the spot is not. Deliver a very small spot of material on the pencil line of the appropriate lane (figure. Explain Why The Chromatography Spot Should Be Small.
From demarion-kcoleman.blogspot.com
During a Chromatography Experiment a Student Calculated Explain Why The Chromatography Spot Should Be Small The silica gel (or the alumina) is. A purple spot should be seen. A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. The spots should be very small, around \(2 \: No, you should always spot the samples slightly above the level of. It is also known as open column. Explain Why The Chromatography Spot Should Be Small.
From animalia-life.club
Paper Chromatography Diagram Explain Why The Chromatography Spot Should Be Small If the spot is not. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot. A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. Using a capillary tube, a. It is also known as open column. Explain Why The Chromatography Spot Should Be Small.
From www.makingmolecules.com
Thin Layer Chromatography (TLC) — Making Molecules Explain Why The Chromatography Spot Should Be Small The spots should be very small, around \(2 \: If the spot is not. Using a capillary tube, a. A purple spot should be seen. Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. The silica gel (or the alumina) is. No, you should always spot the. Explain Why The Chromatography Spot Should Be Small.
From www.mdpi.com
Molecules Free FullText Recent Developments of Liquid Explain Why The Chromatography Spot Should Be Small If the spot is not. Using a capillary tube, a. Should you spot tlc samples right at the bottom of the plate? A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot. Make sure enough sample is spotted on the plate. A chemist often needs to. Explain Why The Chromatography Spot Should Be Small.
From cookinglove.com
How does chromatography separate pigments Explain Why The Chromatography Spot Should Be Small The spots should be very small, around \(2 \: Using a capillary tube, a. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent,. Explain Why The Chromatography Spot Should Be Small.
From jetpaper.web.fc2.com
what industries use chromatography and what do they use this technique for? Explain Why The Chromatography Spot Should Be Small If the spot is not. Deliver a very small spot of material on the pencil line of the appropriate lane (figure 2.25b) using a quick up and. In thin layer chromatography the solid phase (silica gel or alumina) is applied as a thin coating on a plastic sheet or glass slide, called a tlc plate (fig. It is also known. Explain Why The Chromatography Spot Should Be Small.
From www.learnatnoon.com
What is Chromatography? Noon Academy Explain Why The Chromatography Spot Should Be Small Using a capillary tube, a. A purple spot should be seen. Should you spot tlc samples right at the bottom of the plate? A chemist often needs to find the “sweet spot” in regard to solvent polarity to achieve the desired separation. The silica gel (or the alumina) is. In thin layer chromatography the solid phase (silica gel or alumina). Explain Why The Chromatography Spot Should Be Small.
From bitesizebio.com
Column Chromatography Made Simple An Easy to Follow Guide Explain Why The Chromatography Spot Should Be Small The silica gel (or the alumina) is. Make sure enough sample is spotted on the plate. The spots should be very small, around \(2 \: If the spot is not. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent, near one edge, as a small spot. Should you spot tlc samples right. Explain Why The Chromatography Spot Should Be Small.