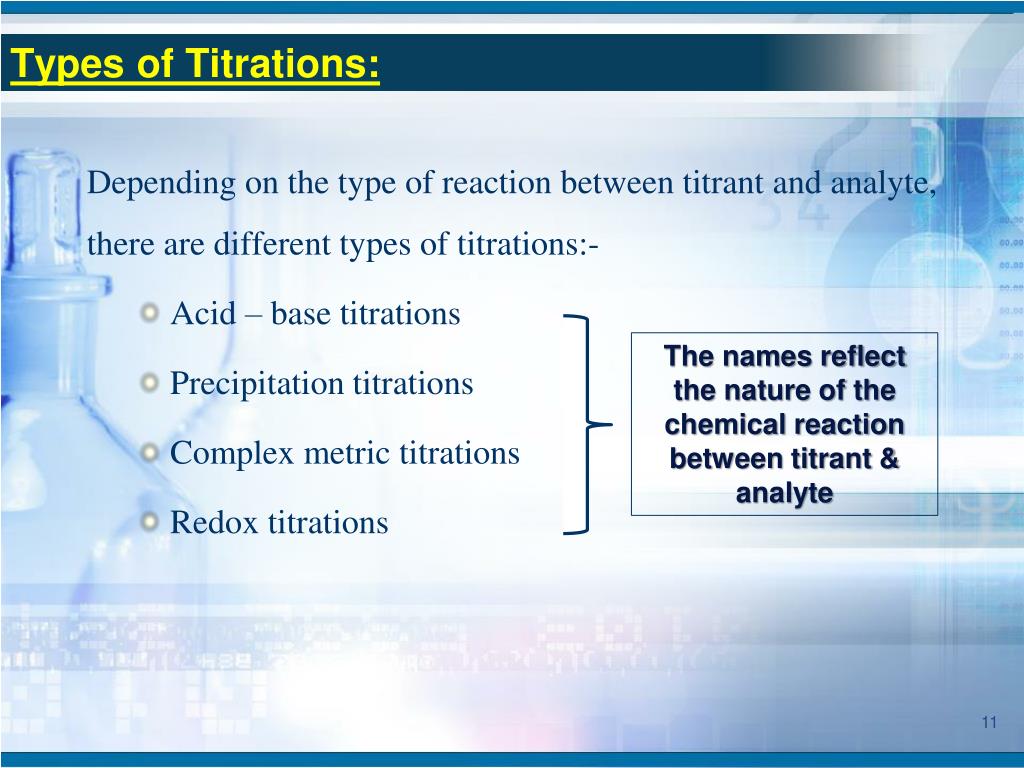
Precipitation Titration Volumetric Analysis . Volhard’s method is used if the ph of the solution in the conical flask is acidic. Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. We call this type of titration a precipitation titration. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. If the solution is neutral or basic, mohr’s method should be used. For example, the concentration of chloride ions in. A precipitation titration is simply one where one of the products of the reaction is a precipitate.
from www.slideserve.com
Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. Volhard’s method is used if the ph of the solution in the conical flask is acidic. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. We call this type of titration a precipitation titration. If the solution is neutral or basic, mohr’s method should be used. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. For example, the concentration of chloride ions in. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. A precipitation titration is simply one where one of the products of the reaction is a precipitate. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a.
PPT Principles of Volumetric (Titrimetric) Analysis PowerPoint
Precipitation Titration Volumetric Analysis Volhard’s method is used if the ph of the solution in the conical flask is acidic. Volhard’s method is used if the ph of the solution in the conical flask is acidic. For example, the concentration of chloride ions in. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. A precipitation titration is simply one where one of the products of the reaction is a precipitate. We call this type of titration a precipitation titration. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. If the solution is neutral or basic, mohr’s method should be used. Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k.
From pediaa.com
Difference Between Gravimetric and Volumetric Analysis Definition Precipitation Titration Volumetric Analysis Volhard’s method is used if the ph of the solution in the conical flask is acidic. We call this type of titration a precipitation titration. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. If the solution is neutral or basic, mohr’s method should. Precipitation Titration Volumetric Analysis.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Precipitation Titration Volumetric Analysis A precipitation titration is simply one where one of the products of the reaction is a precipitate. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. Volhard’s method is used if the ph of the solution in the conical flask is acidic. If the solution is neutral or basic, mohr’s method. Precipitation Titration Volumetric Analysis.
From web.hktechnical.com
Precipitation titrations B.Pharmacy 1st Semester PDF Download 2023 Precipitation Titration Volumetric Analysis Volhard’s method is used if the ph of the solution in the conical flask is acidic. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. A precipitation titration is simply one where one of the products of the reaction is a precipitate. Volumetric or titrimetric analyses are quantitative analytical techniques which employ. Precipitation Titration Volumetric Analysis.
From www.pharmaspecialists.com
Potentiometric Titration in Pharmaceutical Analysis Precipitation Titration Volumetric Analysis If the solution is neutral or basic, mohr’s method should be used. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. A precipitation titration is simply one where one of the products of the reaction is a precipitate. Volumetric or titrimetric analyses are quantitative analytical techniques which. Precipitation Titration Volumetric Analysis.
From www.vrogue.co
Acid Base Titration Volumetric Analysis Slides Pdf Ac vrogue.co Precipitation Titration Volumetric Analysis Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. If the solution is neutral or basic, mohr’s method should be used. One of the earliest precipitation. Precipitation Titration Volumetric Analysis.
From chemistnotes.com
Titration Archives Chemistry Notes Precipitation Titration Volumetric Analysis A precipitation titration is simply one where one of the products of the reaction is a precipitate. Volhard’s method is used if the ph of the solution in the conical flask is acidic. If the solution is neutral or basic, mohr’s method should be used. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is. Precipitation Titration Volumetric Analysis.
From proper-cooking.info
Titration Diagram Precipitation Titration Volumetric Analysis Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique.. Precipitation Titration Volumetric Analysis.
From www.slideshare.net
Precipitation Titration _ Pharmaceutical Analysis _ B. Pharmacy Precipitation Titration Volumetric Analysis One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. For example, the concentration of chloride ions in. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. We call this type of titration a precipitation titration. Precipitation titration is. Precipitation Titration Volumetric Analysis.
From www.studocu.com
Precipitation Titration Notes Pharmaceutical Analysis I Theory Precipitation Titration Volumetric Analysis Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. For example, the concentration of chloride ions in. We call this type of titration a precipitation titration. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. Volhard’s method is used. Precipitation Titration Volumetric Analysis.
From www.slideshare.net
Precipitation Titration _ Pharmaceutical Analysis _ B. Pharmacy Precipitation Titration Volumetric Analysis The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. If the solution is neutral or basic, mohr’s method should be used. For example, the concentration of chloride ions in. We. Precipitation Titration Volumetric Analysis.
From www.slideserve.com
PPT Principles of Volumetric (Titrimetric) Analysis PowerPoint Precipitation Titration Volumetric Analysis If the solution is neutral or basic, mohr’s method should be used. A precipitation titration is simply one where one of the products of the reaction is a precipitate. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. We call this type of titration a precipitation titration.. Precipitation Titration Volumetric Analysis.
From www.studocu.com
Chapter 14 Precipitation titration Precipitation titration Content Precipitation Titration Volumetric Analysis Volhard’s method is used if the ph of the solution in the conical flask is acidic. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. A precipitation titration is simply one where one of the products of the reaction is a precipitate. For example, the concentration of chloride ions in. The. Precipitation Titration Volumetric Analysis.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Precipitation Titration Volumetric Analysis One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. If the solution is neutral or basic, mohr’s method should be used. Volhard’s method is used if the ph of the solution in the conical flask is acidic. Precipitation titration is a type of titration which involves the. Precipitation Titration Volumetric Analysis.
From www.youtube.com
Precipitation Titrations Principle Mohr’s method Volhard’s Precipitation Titration Volumetric Analysis Volhard’s method is used if the ph of the solution in the conical flask is acidic. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. The scale. Precipitation Titration Volumetric Analysis.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Precipitation Titration Volumetric Analysis Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. A precipitation titration is simply one where one of the products of the reaction is a precipitate. Volhard’s method is used if the ph of. Precipitation Titration Volumetric Analysis.
From www.differencebetween.com
Difference Between Gravimetric and Titrimetric Analysis Compare the Precipitation Titration Volumetric Analysis One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation. Precipitation Titration Volumetric Analysis.
From app.emaze.com
types of volumetric copy3 copy1 on emaze Precipitation Titration Volumetric Analysis Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. If the solution is neutral or basic, mohr’s method should be used. For example, the concentration of. Precipitation Titration Volumetric Analysis.
From www.docsity.com
PRECIPITATION TITRATION Lecture notes Pharmaceutical Analysis Docsity Precipitation Titration Volumetric Analysis We call this type of titration a precipitation titration. Volhard’s method is used if the ph of the solution in the conical flask is acidic. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. Precipitation titration is a type of titration which involves the formation of precipitate. Precipitation Titration Volumetric Analysis.
From www.slideserve.com
PPT Principles of Volumetric Analysis PowerPoint Presentation, free Precipitation Titration Volumetric Analysis One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. Precipitation titration is. Precipitation Titration Volumetric Analysis.
From www.chegg.com
Solved An example of a precipitation titration reaction is Precipitation Titration Volumetric Analysis Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. We call this type of titration a precipitation titration. The scale of operations,. Precipitation Titration Volumetric Analysis.
From www.pinterest.com
Lab Report > University of Houston Chem 3369 Chapter0612ac. Good Precipitation Titration Volumetric Analysis For example, the concentration of chloride ions in. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. A precipitation titration is simply one where one of the products of the reaction is a precipitate. Volhard’s. Precipitation Titration Volumetric Analysis.
From www.slideserve.com
PPT AcidBase Titrations A. Definition volumetric analysis of the Precipitation Titration Volumetric Analysis Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique.. Precipitation Titration Volumetric Analysis.
From chem.libretexts.org
9.5 Precipitation Titrations Chemistry LibreTexts Precipitation Titration Volumetric Analysis Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. A precipitation titration is simply one where one of the products of the reaction is a precipitate. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. Precipitation titration is an analytical chemistry technique that depends. Precipitation Titration Volumetric Analysis.
From www.zogirls.com
Performing a Titration & Volumetric Analysis (4.1.2) AQA A Level Precipitation Titration Volumetric Analysis A precipitation titration is simply one where one of the products of the reaction is a precipitate. If the solution is neutral or basic, mohr’s method should be used. For example, the concentration of chloride ions in. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in. Precipitation Titration Volumetric Analysis.
From www.vrogue.co
Ppt Exp 13 Volumetric Analysis Acid Base Titration Po vrogue.co Precipitation Titration Volumetric Analysis Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. Volhard’s method is used if the ph of the solution in the conical flask is acidic. We call this type of titration a precipitation titration. For example, the concentration of chloride ions in. Volumetric or. Precipitation Titration Volumetric Analysis.
From chem.libretexts.org
9.5 Precipitation Titrations Chemistry LibreTexts Precipitation Titration Volumetric Analysis Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. For example, the concentration of chloride ions in. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a.. Precipitation Titration Volumetric Analysis.
From www.scribd.com
Precipitation Titrations Precipitation (Chemistry) Titration Precipitation Titration Volumetric Analysis Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. We call this type of titration a precipitation titration. A precipitation titration is simply one where one. Precipitation Titration Volumetric Analysis.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube Precipitation Titration Volumetric Analysis For example, the concentration of chloride ions in. We call this type of titration a precipitation titration. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. If the solution is neutral or basic, mohr’s method should be used. A precipitation titration is simply one where one of the products of the. Precipitation Titration Volumetric Analysis.
From www.blackdragonrevision.co.uk
ALevel Chemistry Revision Cards Precipitation Titration Volumetric Analysis The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. If the solution is neutral or basic, mohr’s method should be used. We call this type of titration a precipitation titration. Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. For example, the concentration. Precipitation Titration Volumetric Analysis.
From www.chegg.com
Solved 7) A Mohr titration is a precipitation titration used Precipitation Titration Volumetric Analysis Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. Volhard’s method is used if the ph of the solution in the conical flask is acidic. If the. Precipitation Titration Volumetric Analysis.
From www.scribd.com
10 Precipitation Titration PDF Solubility Precipitation (Chemistry) Precipitation Titration Volumetric Analysis Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. We call this type of titration a precipitation titration. A precipitation titration is simply one where one of the products of the reaction is a precipitate. For example, the concentration of chloride ions in. Volumetric or titrimetric analyses are quantitative analytical techniques which. Precipitation Titration Volumetric Analysis.
From www.slideserve.com
PPT Ch 6 Precipitation Titrations, Sec 65 and 66 PowerPoint Precipitation Titration Volumetric Analysis Volhard’s method is used if the ph of the solution in the conical flask is acidic. Precipitation titration is a type of titration which involves the formation of precipitate during the titration technique. The scale of operations, accuracy, precision, sensitivity, time, and cost of a precipitation titration is similar to. Volumetric or titrimetric analyses are quantitative analytical techniques which employ. Precipitation Titration Volumetric Analysis.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Precipitation Titration Volumetric Analysis One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. If the solution is neutral or basic, mohr’s method should be used. Precipitation titration is an analytical chemistry technique that depends on the formation of a precipitate to determine the concentration of an analyte in a solution. We. Precipitation Titration Volumetric Analysis.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions Precipitation Titration Volumetric Analysis Volumetric or titrimetric analyses are quantitative analytical techniques which employ a titration in comparing an unknown with a. Volhard’s method is used if the ph of the solution in the conical flask is acidic. A precipitation titration is simply one where one of the products of the reaction is a precipitate. The scale of operations, accuracy, precision, sensitivity, time, and. Precipitation Titration Volumetric Analysis.
From www.youtube.com
Precipitation Titrations YouTube Precipitation Titration Volumetric Analysis For example, the concentration of chloride ions in. Volhard’s method is used if the ph of the solution in the conical flask is acidic. One of the earliest precipitation titrations—developed at the end of the eighteenth century—was the analysis of k 2 co 3 and k. Precipitation titration is an analytical chemistry technique that depends on the formation of a. Precipitation Titration Volumetric Analysis.