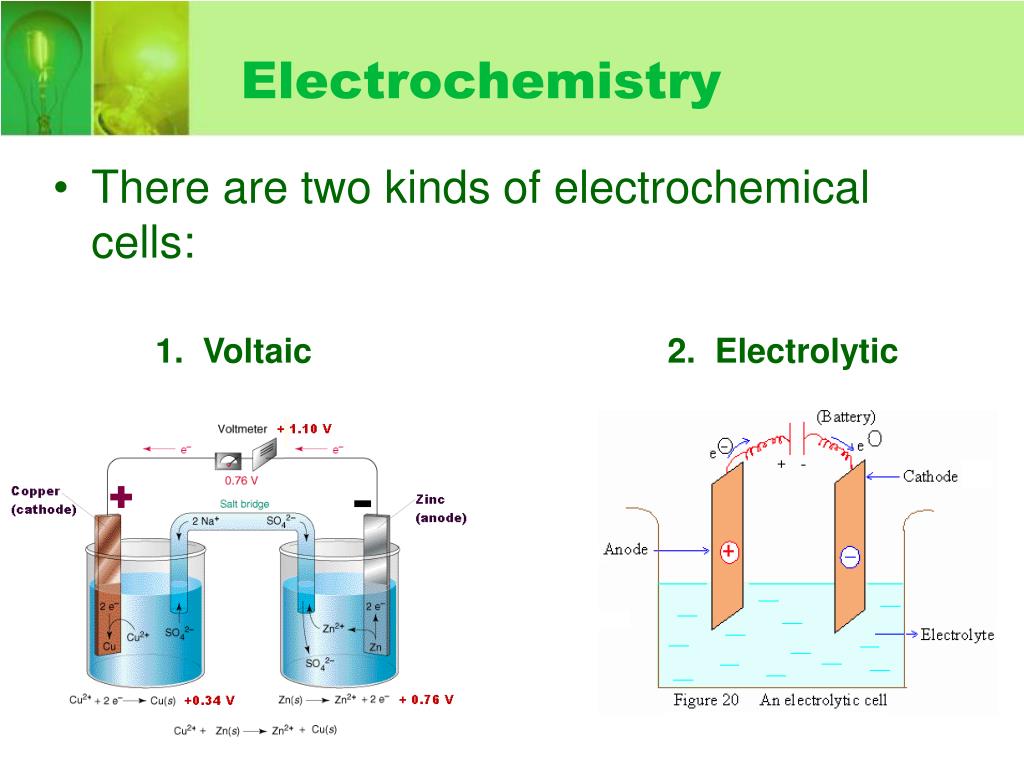
Types Of Electrodes In Electrochemistry Ppt . Electrode at which reduction occurs. Potential of a cell composed of a working electrode (1 m. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Each electrode along with the electrolyte constitutes a half. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. This document provides an overview of electrochemistry. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. It begins by defining electrochemistry as the study of chemical. Potential difference that is the driving force that propels electrons from the. The study of chemical reactions that involve electron or charge transfer (e.g., ions).
from www.slideserve.com
It begins by defining electrochemistry as the study of chemical. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Electrode at which reduction occurs. This document provides an overview of electrochemistry. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Each electrode along with the electrolyte constitutes a half. Potential of a cell composed of a working electrode (1 m. The study of chemical reactions that involve electron or charge transfer (e.g., ions). Potential difference that is the driving force that propels electrons from the.
PPT Electrochemistry PowerPoint Presentation, free download ID5570772
Types Of Electrodes In Electrochemistry Ppt Each electrode along with the electrolyte constitutes a half. This document provides an overview of electrochemistry. It begins by defining electrochemistry as the study of chemical. Each electrode along with the electrolyte constitutes a half. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Potential of a cell composed of a working electrode (1 m. Potential difference that is the driving force that propels electrons from the. Electrode at which reduction occurs. The study of chemical reactions that involve electron or charge transfer (e.g., ions). This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt It begins by defining electrochemistry as the study of chemical. This document provides an overview of electrochemistry. Potential of a cell composed of a working electrode (1 m. Each electrode along with the electrolyte constitutes a half. Electrode at which reduction occurs. Potential difference that is the driving force that propels electrons from the. This document discusses key concepts in. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. It begins by defining electrochemistry as the study of chemical. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Potential difference that is the driving force that propels electrons from the. This chapter introduces. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt Electrode at which reduction occurs. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. It begins by defining electrochemistry as the study of chemical. Each electrode along with the electrolyte constitutes a half. Potential difference that is the driving force that propels electrons from the. Different types of electrodes. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. Each electrode along with the electrolyte constitutes a half. The study of chemical reactions that involve electron or charge transfer (e.g., ions). Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place.. Types Of Electrodes In Electrochemistry Ppt.
From ppt-online.org
Electrolysis презентация онлайн Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. Potential of a cell composed of a working electrode (1 m. Potential difference that is the driving force that propels electrons from the. The study of chemical reactions that involve electron or charge transfer (e.g., ions). Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. Electrode at. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3079461 Types Of Electrodes In Electrochemistry Ppt Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. Potential difference that is the driving force that propels electrons from the. It begins by defining electrochemistry as the study of chemical. The study of chemical reactions that involve electron or charge transfer (e.g., ions). Potential of a cell composed of a working electrode (1. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt Potential of a cell composed of a working electrode (1 m. Electrode at which reduction occurs. This document provides an overview of electrochemistry. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Different. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt It begins by defining electrochemistry as the study of chemical. Electrode at which reduction occurs. Each electrode along with the electrolyte constitutes a half. Potential of a cell composed of a working electrode (1 m. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. The study of chemical reactions that involve electron or. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Chapter 10 Equilibrium Electrochemistry PowerPoint Presentation ID1269991 Types Of Electrodes In Electrochemistry Ppt This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. Potential difference that is the driving force that propels electrons from the. The study of chemical reactions that involve electron or charge transfer (e.g., ions).. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free download ID3358415 Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. Potential of a cell composed of a working electrode (1 m. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Potential difference that is the driving force that propels electrons from the. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. Potential of a cell composed of a working electrode (1 m. Each electrode along with the electrolyte constitutes a half. The study of chemical reactions that involve electron or charge transfer (e.g., ions). It begins by defining electrochemistry. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT electrochemistry PowerPoint Presentation ID2683693 Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. It begins by defining electrochemistry as the study of chemical. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Potential difference that is the driving force that propels electrons from the.. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Types of Electrochemical Cells PowerPoint Presentation, free download ID3222493 Types Of Electrodes In Electrochemistry Ppt Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Electrode at which reduction occurs. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Each electrode along with. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT 5. Electrochemistry Ion Selective Electrodes Anodic Stripping Voltammetry PowerPoint Types Of Electrodes In Electrochemistry Ppt The study of chemical reactions that involve electron or charge transfer (e.g., ions). Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. Potential difference that is the driving force that propels electrons from the. Each electrode along with the electrolyte constitutes a half. This document provides an overview of electrochemistry. This document discusses key. Types Of Electrodes In Electrochemistry Ppt.
From scienceinfo.com
Electrochemistry Definition, Types, Components Types Of Electrodes In Electrochemistry Ppt It begins by defining electrochemistry as the study of chemical. Potential of a cell composed of a working electrode (1 m. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. The study of chemical reactions that involve electron or charge transfer (e.g., ions). Different types of electrodes • an. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Types Of Electrodes In Electrochemistry Ppt This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Electrode at which reduction occurs. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. Potential of a cell. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free download ID3358415 Types Of Electrodes In Electrochemistry Ppt The study of chemical reactions that involve electron or charge transfer (e.g., ions). Potential of a cell composed of a working electrode (1 m. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. Potential difference that is the. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt Each electrode along with the electrolyte constitutes a half. Potential difference that is the driving force that propels electrons from the. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. The study of chemical reactions that involve electron or charge transfer (e.g., ions). This document provides an overview of electrochemistry. This chapter introduces the. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt Each electrode along with the electrolyte constitutes a half. Potential difference that is the driving force that propels electrons from the. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Electrode at which. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5570772 Types Of Electrodes In Electrochemistry Ppt This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Electrode at which reduction occurs. Potential of a cell composed of a working electrode (1 m. The study of chemical reactions that involve electron or charge transfer (e.g., ions). This chapter introduces the electrochemical cell, its components, basic definitions, and. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5570772 Types Of Electrodes In Electrochemistry Ppt Electrode at which reduction occurs. Potential difference that is the driving force that propels electrons from the. Each electrode along with the electrolyte constitutes a half. This document provides an overview of electrochemistry. Potential of a cell composed of a working electrode (1 m. The study of chemical reactions that involve electron or charge transfer (e.g., ions). This chapter introduces. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3079461 Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. Electrode at which reduction occurs. Potential of a cell composed of a working electrode (1 m. It begins by defining electrochemistry as the study of chemical. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Each electrode along with the electrolyte constitutes a half. Different types. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt Potential difference that is the driving force that propels electrons from the. This document provides an overview of electrochemistry. Electrode at which reduction occurs. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Each electrode along with the electrolyte constitutes a half. It begins by defining electrochemistry as the. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. This document provides an overview of electrochemistry. Electrode at which reduction occurs. Each electrode along with the electrolyte constitutes a half. Potential difference that. Types Of Electrodes In Electrochemistry Ppt.
From greenenergymaterial.com
Types Of Electrodes In Electrochemistry » Green Energy Material Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. Each electrode along with the electrolyte constitutes a half. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. It begins by defining electrochemistry as the study of chemical. Potential of a cell composed of a working electrode (1 m. This chapter introduces. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt The study of chemical reactions that involve electron or charge transfer (e.g., ions). Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. This document provides an overview of electrochemistry. Potential of a cell composed of a working electrode. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. It begins by defining electrochemistry as the study of chemical. Potential difference that is the driving force that propels electrons from the. Each electrode along with the electrolyte constitutes a half. Electrode at. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3079461 Types Of Electrodes In Electrochemistry Ppt Potential difference that is the driving force that propels electrons from the. It begins by defining electrochemistry as the study of chemical. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Each electrode along with the electrolyte constitutes a half. The study of chemical reactions that involve electron or. Types Of Electrodes In Electrochemistry Ppt.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry. YouTube Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. Potential of a cell composed of a working electrode (1 m. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Each electrode along with the electrolyte constitutes a half. This document. Types Of Electrodes In Electrochemistry Ppt.
From www.youtube.com
What are different types of Reversible Electrodes? Electrochemistry Physical Chemistry YouTube Types Of Electrodes In Electrochemistry Ppt This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. Potential of a cell composed of a working electrode (1 m. The study of chemical reactions that involve electron or charge transfer (e.g., ions). Each electrode along with the electrolyte constitutes a half. This chapter introduces the electrochemical cell, its. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Types Of Electrodes In Electrochemistry Ppt This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Potential of a cell composed of a working electrode (1 m. The study of chemical reactions that involve electron or charge transfer (e.g., ions). It begins by defining electrochemistry as the study of chemical. Each electrode along with the electrolyte constitutes a half. This. Types Of Electrodes In Electrochemistry Ppt.
From pdfslide.net
(PPT) Electrochemistry Plan 1. Electrode processes. Electrode potential. 2. Different types of Types Of Electrodes In Electrochemistry Ppt Potential of a cell composed of a working electrode (1 m. This document provides an overview of electrochemistry. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. Potential difference that is the driving force that propels electrons from the. It begins by defining electrochemistry as the study of chemical. Electrode at which reduction. Types Of Electrodes In Electrochemistry Ppt.
From www.slideshare.net
Chapter 6 electrochemistry Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. Electrode at which reduction occurs. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. This chapter introduces the electrochemical cell, its components, basic definitions, and the processes that take place. It begins by defining electrochemistry as the study of chemical. Different types. Types Of Electrodes In Electrochemistry Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Types Of Electrodes In Electrochemistry Ppt This document provides an overview of electrochemistry. It begins by defining electrochemistry as the study of chemical. Potential of a cell composed of a working electrode (1 m. Electrode at which reduction occurs. Potential difference that is the driving force that propels electrons from the. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics. Types Of Electrodes In Electrochemistry Ppt.
From www.youtube.com
Electrochemistry 6 Type of Electrodes YouTube Types Of Electrodes In Electrochemistry Ppt Potential difference that is the driving force that propels electrons from the. Potential of a cell composed of a working electrode (1 m. Different types of electrodes • an electrochemical cell consists of two electrodes, positive and negative. This document discusses key concepts in electrochemistry including the interface between electrode and electrolyte, thermodynamics and kinetics of electrode reactions,. The study. Types Of Electrodes In Electrochemistry Ppt.