Copper Oxide React With Water . The metal oxides form alkaline solutions in water, the oxides in the middle do not. They will react with water to produce acidic solutions examiner tip key thing to remember: Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. The reaction between a metal oxide and acid is an example of a neutralization reaction. 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. As a result, hydroxide ion can displace water from the. In a neutralization reaction, an acid reacts with a.
from www.youtube.com
They will react with water to produce acidic solutions examiner tip key thing to remember: The reaction between a metal oxide and acid is an example of a neutralization reaction. As a result, hydroxide ion can displace water from the. In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. The metal oxides form alkaline solutions in water, the oxides in the middle do not. In a neutralization reaction, an acid reacts with a.
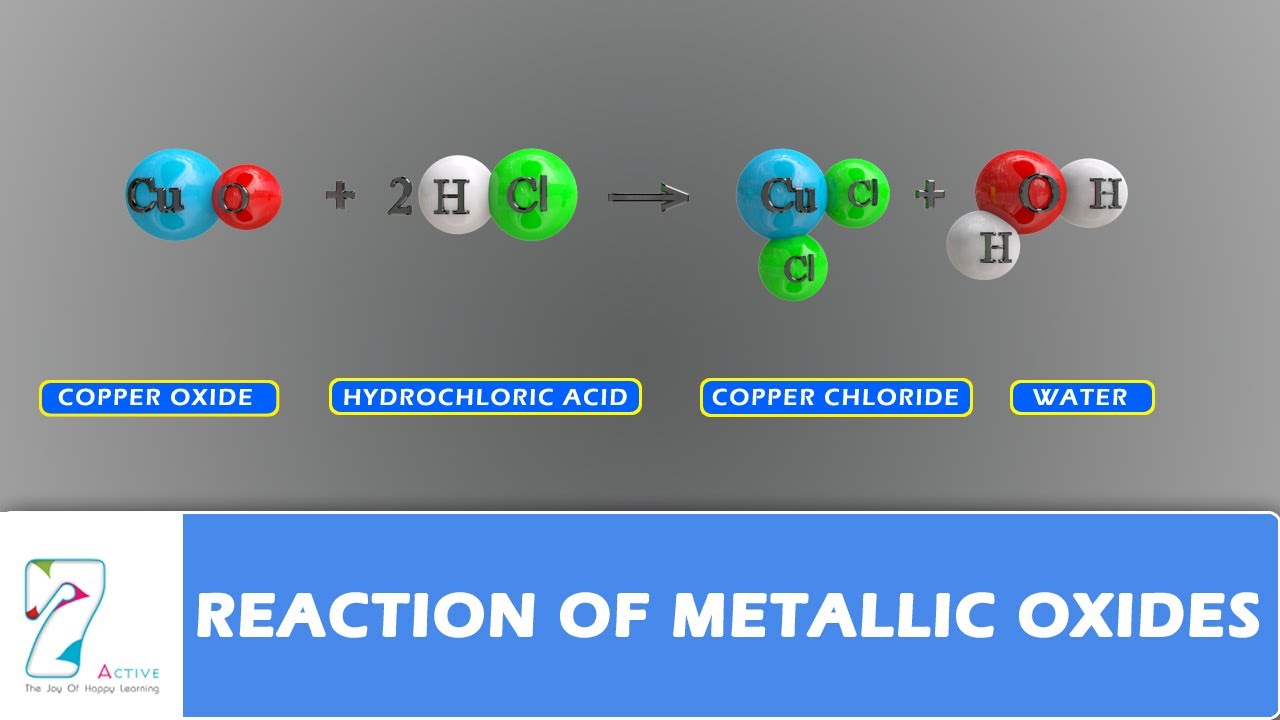
Reaction of Metallic oxides YouTube
Copper Oxide React With Water They will react with water to produce acidic solutions examiner tip key thing to remember: The reaction between a metal oxide and acid is an example of a neutralization reaction. In a neutralization reaction, an acid reacts with a. The metal oxides form alkaline solutions in water, the oxides in the middle do not. 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. As a result, hydroxide ion can displace water from the. Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. They will react with water to produce acidic solutions examiner tip key thing to remember: In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts:
From www.youtube.com
Hydrochloric Acid Reaction HCL and mixed metal copper oxides YouTube Copper Oxide React With Water Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. They will react with water to produce acidic solutions examiner tip key thing to remember: In a neutralization reaction, an acid reacts with a. The reaction between a metal oxide and acid is an example of a neutralization reaction. As. Copper Oxide React With Water.
From fineartamerica.com
Two Copper Oxide Reactions Photograph by Andrew Lambert Photography Copper Oxide React With Water Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: The reaction between a metal oxide and acid is an example of a neutralization reaction. They will react with water to produce acidic solutions examiner. Copper Oxide React With Water.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Copper Oxide React With Water 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: They will react with water to produce acidic solutions examiner tip key thing to remember: Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper. Copper Oxide React With Water.
From www.youtube.com
Cu + H2O (Copper plus Water) YouTube Copper Oxide React With Water 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: The reaction between a metal oxide and acid is an example of. Copper Oxide React With Water.
From www.alamy.com
copper oxide and sulphuric acid form copper sulphate solution which is Copper Oxide React With Water Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. The reaction between a metal oxide and acid is an example of a neutralization reaction. The metal oxides form alkaline solutions in water, the oxides in the middle do not. They will react with water to produce acidic solutions examiner tip key thing. Copper Oxide React With Water.
From www.youtube.com
Reaction of Metallic oxides YouTube Copper Oxide React With Water As a result, hydroxide ion can displace water from the. Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. In a neutralization reaction, an acid reacts with a. Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. 2 naoh + cuo. Copper Oxide React With Water.
From www.youtube.com
How to Balance CuO + H2 = Cu + H2O Copper (II) Oxide and Hydrogen Gas Copper Oxide React With Water Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. They will react with water to produce acidic solutions examiner tip key thing to remember: 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. The metal oxides form alkaline solutions in water, the. Copper Oxide React With Water.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Oxide React With Water In a neutralization reaction, an acid reacts with a. Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: They will react with water to produce acidic solutions examiner tip key thing to remember: Copper. Copper Oxide React With Water.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Oxide React With Water In a neutralization reaction, an acid reacts with a. The metal oxides form alkaline solutions in water, the oxides in the middle do not. They will react with water to produce acidic solutions examiner tip key thing to remember: The reaction between a metal oxide and acid is an example of a neutralization reaction. Copper (i) oxide can react with. Copper Oxide React With Water.
From slideplayer.com
1. ppt download Copper Oxide React With Water 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. The reaction between a metal oxide and acid is an example of a neutralization reaction. Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. In presence of water it reacts with concentrated alkali. Copper Oxide React With Water.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Copper Oxide React With Water Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. They will react with water to produce acidic solutions examiner tip key thing to remember: 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. Copper hydroxide and copper carbonate are insoluble in water,. Copper Oxide React With Water.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID7032715 Copper Oxide React With Water The metal oxides form alkaline solutions in water, the oxides in the middle do not. The reaction between a metal oxide and acid is an example of a neutralization reaction. In a neutralization reaction, an acid reacts with a. They will react with water to produce acidic solutions examiner tip key thing to remember: In presence of water it reacts. Copper Oxide React With Water.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II Copper Oxide React With Water In a neutralization reaction, an acid reacts with a. The metal oxides form alkaline solutions in water, the oxides in the middle do not. Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. The reaction between a metal oxide and acid is an example of a neutralization reaction. 2 naoh + cuo. Copper Oxide React With Water.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper Oxide React With Water The reaction between a metal oxide and acid is an example of a neutralization reaction. As a result, hydroxide ion can displace water from the. 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. They will react with water to produce acidic solutions examiner tip key thing to remember: In presence of water. Copper Oxide React With Water.
From askfilo.com
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We.. Copper Oxide React With Water Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. In a neutralization reaction, an acid reacts with a. As a result, hydroxide ion can displace water from the. The reaction between a metal oxide and acid is an example of a neutralization reaction. Copper (i) oxide can react with water as the. Copper Oxide React With Water.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper Oxide React With Water Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. The reaction between a metal oxide and acid is an example of a neutralization reaction. The metal oxides form alkaline solutions in water, the oxides in the middle do not. In presence of water it reacts with concentrated alkali to. Copper Oxide React With Water.
From byjus.com
1 what quantity of copper oxide will react with 2.8l of hydrogen at ntp Copper Oxide React With Water The metal oxides form alkaline solutions in water, the oxides in the middle do not. In a neutralization reaction, an acid reacts with a. Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. The reaction between. Copper Oxide React With Water.
From www.mdpi.com
Sustainable Chemistry Free FullText Recent Advances in Copper Copper Oxide React With Water As a result, hydroxide ion can displace water from the. The metal oxides form alkaline solutions in water, the oxides in the middle do not. In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide.. Copper Oxide React With Water.
From www.youtube.com
neutralisation of sulphuric acid with copper oxide.mp4 YouTube Copper Oxide React With Water In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. The reaction between a metal oxide and acid is an example of a neutralization reaction. They will react with water to produce acidic solutions examiner tip key thing. Copper Oxide React With Water.
From byjus.com
Copper reacts with dilute nitric acid and forms copper nitrate, nitric Copper Oxide React With Water As a result, hydroxide ion can displace water from the. They will react with water to produce acidic solutions examiner tip key thing to remember: In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. In a neutralization reaction,. Copper Oxide React With Water.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper Oxide React With Water In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. The reaction between a metal oxide and acid. Copper Oxide React With Water.
From chemicaldb.netlify.app
Why does copper oxide not dissolve in water Copper Oxide React With Water Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. In a neutralization reaction, an acid reacts with a. 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. As a result, hydroxide ion can displace water from the. The reaction between a metal oxide and acid. Copper Oxide React With Water.
From www.doubtnut.com
When hydrogen gas is passed over heated copper (II) oxide, copper and Copper Oxide React With Water The metal oxides form alkaline solutions in water, the oxides in the middle do not. Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. As a result, hydroxide ion can displace water from the. The reaction between a metal oxide and acid is an example of a neutralization reaction. In a neutralization. Copper Oxide React With Water.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Oxide React With Water Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. In a neutralization reaction, an acid reacts with a. 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4]. Copper Oxide React With Water.
From www.youtube.com
Mixing copper oxide with distilled water and sulfuric acid MVI 6706 Copper Oxide React With Water As a result, hydroxide ion can displace water from the. 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. In a neutralization reaction, an acid reacts with a. Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. In presence of water it reacts with concentrated. Copper Oxide React With Water.
From www.youtube.com
Heating a mixture of carbon and copper (II) oxide YouTube Copper Oxide React With Water 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. They will react with water to produce acidic solutions examiner tip key thing to remember: As a result, hydroxide ion can displace water from the. The reaction between a metal oxide and acid is an example of a neutralization reaction. The metal oxides form. Copper Oxide React With Water.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Copper Oxide React With Water 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. The metal oxides form alkaline solutions in water, the oxides in the middle do not. They will react with water to produce acidic solutions examiner tip key thing to remember: As a result, hydroxide ion can displace water from the. The reaction between a. Copper Oxide React With Water.
From www.youtube.com
How to Balance Na2SO4 + C = Na2S + CO2 YouTube Copper Oxide React With Water Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. The metal oxides form alkaline solutions in water, the oxides in the middle do not. They will react with water to produce acidic. Copper Oxide React With Water.
From www.youtube.com
Copper oxide react with Hydrochloride Experiment / Experiment Copper Oxide React With Water Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. They will react with water to produce acidic solutions examiner tip key thing to remember: In a neutralization reaction, an acid reacts with a. The metal oxides form alkaline solutions in water, the oxides in the middle do not. Copper. Copper Oxide React With Water.
From www.numerade.com
SOLVED Ammonia gas and solid copper(II) oxide react to form liquid Copper Oxide React With Water They will react with water to produce acidic solutions examiner tip key thing to remember: The reaction between a metal oxide and acid is an example of a neutralization reaction. In a neutralization reaction, an acid reacts with a. Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. The. Copper Oxide React With Water.
From trystan-has-mahoney.blogspot.com
Is the Reactivity of Copper With Oxygen a Chemical Property Trystan Copper Oxide React With Water The reaction between a metal oxide and acid is an example of a neutralization reaction. They will react with water to produce acidic solutions examiner tip key thing to remember: Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. In presence of water it reacts with concentrated alkali to form the corresponding. Copper Oxide React With Water.
From www.youtube.com
Reaction of copper oxide with Hcl YouTube Copper Oxide React With Water As a result, hydroxide ion can displace water from the. In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: Copper hydroxide and copper carbonate are insoluble in water, but copper sulphate and copper chloride moderately enter. The reaction between a metal oxide and acid is an example of a neutralization reaction. 2 naoh +. Copper Oxide React With Water.
From www.youtube.com
Copper Oxide Synthesis YouTube Copper Oxide React With Water The metal oxides form alkaline solutions in water, the oxides in the middle do not. Copper (i) oxide can react with water as the oxygen is present in the water and make copper (ii) hydroxide. In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: Copper hydroxide and copper carbonate are insoluble in water, but. Copper Oxide React With Water.
From www.sciencephoto.com
Copper (II) oxide reacts with hydrochloric acid Stock Image C036 Copper Oxide React With Water 2 naoh + cuo + h 2 o → na 2 [cu (oh) 4] it can. The metal oxides form alkaline solutions in water, the oxides in the middle do not. They will react with water to produce acidic solutions examiner tip key thing to remember: In presence of water it reacts with concentrated alkali to form the corresponding cuprate. Copper Oxide React With Water.
From www.numerade.com
Ammonia and copper (II) oxide react according to the following equation Copper Oxide React With Water In presence of water it reacts with concentrated alkali to form the corresponding cuprate salts: They will react with water to produce acidic solutions examiner tip key thing to remember: The metal oxides form alkaline solutions in water, the oxides in the middle do not. As a result, hydroxide ion can displace water from the. Copper (i) oxide can react. Copper Oxide React With Water.