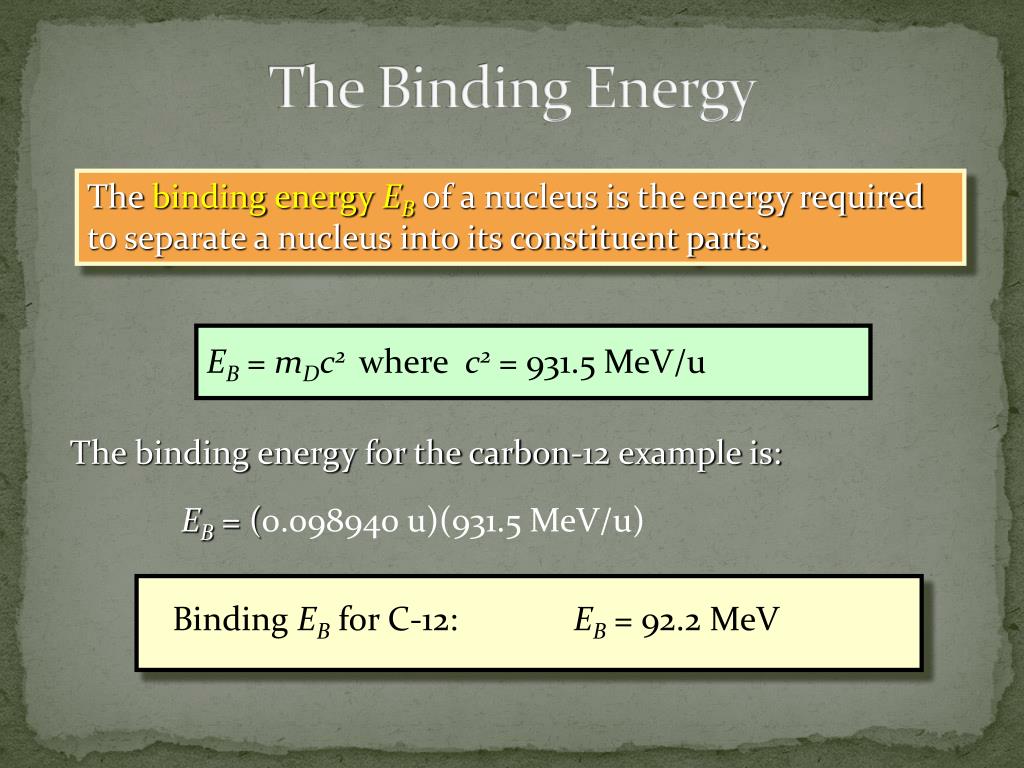
What Does Higher Binding Energy Mean . The mass of an atomic. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The smaller the size of a bound. This quantity is the average energy. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. 7 rows there are several types of binding energy, each operating over a different distance and energy scale. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts.
from www.slideserve.com
In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. This quantity is the average energy. 7 rows there are several types of binding energy, each operating over a different distance and energy scale. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. The mass of an atomic. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. The smaller the size of a bound. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion.
PPT Nuclear Physics PowerPoint Presentation, free download ID1985809
What Does Higher Binding Energy Mean In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. This quantity is the average energy. The mass of an atomic. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. The smaller the size of a bound. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. 7 rows there are several types of binding energy, each operating over a different distance and energy scale. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo.
From whatsinsight.org
Nuclear Binding Energy Formula Step By Step Calculation What's Insight What Does Higher Binding Energy Mean 7 rows there are several types of binding energy, each operating over a different distance and energy scale. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. Electron binding energy, also called ionization potential, is the energy required to remove an electron from. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT 26.1 Properties of nucleus 26.2 Binding energy and mass defect What Does Higher Binding Energy Mean Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. The smaller the size of a bound. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. The mass of an. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Binding Energy PowerPoint Presentation, free download ID1448261 What Does Higher Binding Energy Mean The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. This quantity is the average energy. The smaller the size of a bound. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Electron binding energy, also called ionization potential,. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Nuclear Binding Energy PowerPoint Presentation, free download What Does Higher Binding Energy Mean This quantity is the average energy. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. The mass of an atomic. A high binding energy. What Does Higher Binding Energy Mean.
From www.youtube.com
Class XII Binding energy curve YouTube What Does Higher Binding Energy Mean Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. The mass of an atomic. The smaller the size of a bound. This quantity is the average energy. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT CHAPTER 30 PowerPoint Presentation, free download ID1985636 What Does Higher Binding Energy Mean Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. In nuclear physics, one of the most important experimental quantities is the. What Does Higher Binding Energy Mean.
From byjus.com
Why Fe has highest binding energy and the binding energy curve What Does Higher Binding Energy Mean Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. 7 rows there are several types of binding energy, each operating over a different distance and energy scale. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons.. What Does Higher Binding Energy Mean.
From www.youtube.com
C.7 Calculating binding energy (HL) YouTube What Does Higher Binding Energy Mean This quantity is the average energy. 7 rows there are several types of binding energy, each operating over a different distance and energy scale. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. The mass of an atomic. Nuclear binding energy, the energy required to. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Binding Energy PowerPoint Presentation, free download ID1150105 What Does Higher Binding Energy Mean Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. 7 rows there are several types of binding energy, each operating over a different distance. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Fission and fusion PowerPoint Presentation, free download ID What Does Higher Binding Energy Mean The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. The mass of an atomic. Electron binding energy, also called ionization potential, is the energy required to. What Does Higher Binding Energy Mean.
From byjus.com
What do you mean by binding energy? What Does Higher Binding Energy Mean The smaller the size of a bound. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. In nuclear physics, one of the most important. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Binding Energy PowerPoint Presentation, free download ID1150105 What Does Higher Binding Energy Mean The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The smaller the size of a bound. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. A high binding energy indicates a stable nucleus, while a low binding. What Does Higher Binding Energy Mean.
From www.energyencyclopedia.com
Binding energy curve Images Free Downloads Energy Encyclopedia What Does Higher Binding Energy Mean The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may. What Does Higher Binding Energy Mean.
From general.chemistrysteps.com
Nuclear Binding Energy Chemistry Steps What Does Higher Binding Energy Mean In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. This quantity is the average energy. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. The mass of an atomic. 7 rows there are. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Nuclear Binding Energy PowerPoint Presentation, free download What Does Higher Binding Energy Mean Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. A high binding energy indicates a stable nucleus, while a low binding. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Nuclear Physics PowerPoint Presentation, free download ID1985809 What Does Higher Binding Energy Mean A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. The smaller the size of a bound. 7 rows there are several types of binding energy, each operating over a different distance and energy scale. In nuclear physics, one of the most important experimental quantities is. What Does Higher Binding Energy Mean.
From www.researchgate.net
(a) XPS binding energy spectra of Tipeak shifting and highresolution What Does Higher Binding Energy Mean In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. This quantity is the average energy. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. 7 rows there are several types of. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Nuclear Binding Energy PowerPoint Presentation, free download What Does Higher Binding Energy Mean A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. The mass of an atomic. This quantity is the average energy. The binding. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Chapter 29 PowerPoint Presentation, free download ID4762406 What Does Higher Binding Energy Mean In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. A high binding energy indicates a stable nucleus, while a low binding energy. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Binding Energy PowerPoint Presentation, free download ID1150105 What Does Higher Binding Energy Mean A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. 7 rows there are several types of binding energy, each operating over a different distance and energy scale. The mass of an atomic. The binding energy (be) of a nucleus is the energy needed to separate. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Binding energy in atoms and nuclei PowerPoint Presentation, free What Does Higher Binding Energy Mean The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Lecture 27 Nuclear Reactions PowerPoint Presentation, free What Does Higher Binding Energy Mean Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. This quantity is the average energy. The smaller the size of a bound. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo.. What Does Higher Binding Energy Mean.
From study.com
Binding Energy Curves & Nuclear Energy Lesson What Does Higher Binding Energy Mean The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The mass of an atomic. The smaller the size of a bound. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Nuclear binding energy is the minimum. What Does Higher Binding Energy Mean.
From byjus.com
Definition of binding energy What Does Higher Binding Energy Mean Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. The smaller the size of a bound. Nuclear binding energy, the energy required to separate an atomic. What Does Higher Binding Energy Mean.
From eduinput.com
What is Binding Energy?Definition, Types, And Applications What Does Higher Binding Energy Mean This quantity is the average energy. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. A high binding energy indicates a. What Does Higher Binding Energy Mean.
From curiophysics.com
Binding Energy Per Nucleon Binding Energy Curve » Curio Physics What Does Higher Binding Energy Mean 7 rows there are several types of binding energy, each operating over a different distance and energy scale. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Introduction PowerPoint Presentation, free download ID6535249 What Does Higher Binding Energy Mean The smaller the size of a bound. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. The binding energy (be) of. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Binding Energy PowerPoint Presentation, free download ID1150105 What Does Higher Binding Energy Mean The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or,. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Nuclear Physics PowerPoint Presentation, free download ID2238937 What Does Higher Binding Energy Mean 7 rows there are several types of binding energy, each operating over a different distance and energy scale. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone. What Does Higher Binding Energy Mean.
From www.universetoday.com
What is Binding Energy? Universe Today What Does Higher Binding Energy Mean A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Electron binding energy, also called ionization potential, is the energy required to remove an electron. What Does Higher Binding Energy Mean.
From www.youtube.com
Nuclear Binding Energy tutorial (Post 16 physics) YouTube What Does Higher Binding Energy Mean This quantity is the average energy. Nuclear binding energy, the energy required to separate an atomic nucleus completely into its constituent protons and neutrons, or, equivalently, the energy that would be liberated. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. The binding energy (be) of a nucleus. What Does Higher Binding Energy Mean.
From www.youtube.com
HTPIB30O The Curve of Binding Energy YouTube What Does Higher Binding Energy Mean A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may be prone to decay or undergo. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. This quantity is the average energy. 7 rows there are several types of. What Does Higher Binding Energy Mean.
From www.slideserve.com
PPT Nuclear Binding Energy PowerPoint Presentation, free download What Does Higher Binding Energy Mean Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. A high binding energy indicates a stable nucleus, while a low binding energy suggests that the nucleus may. What Does Higher Binding Energy Mean.
From www.britannica.com
Nuclear binding energy Definition, Formula, Mass Defect, & Graph What Does Higher Binding Energy Mean This quantity is the average energy. The mass of an atomic. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. 7 rows there are several types of. What Does Higher Binding Energy Mean.
From www.vedantu.com
What is the significance of binding energy per nucleon of a nucleus? What Does Higher Binding Energy Mean The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. This quantity is the average energy. Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. Nuclear binding energy, the energy required to separate an atomic nucleus completely into. What Does Higher Binding Energy Mean.