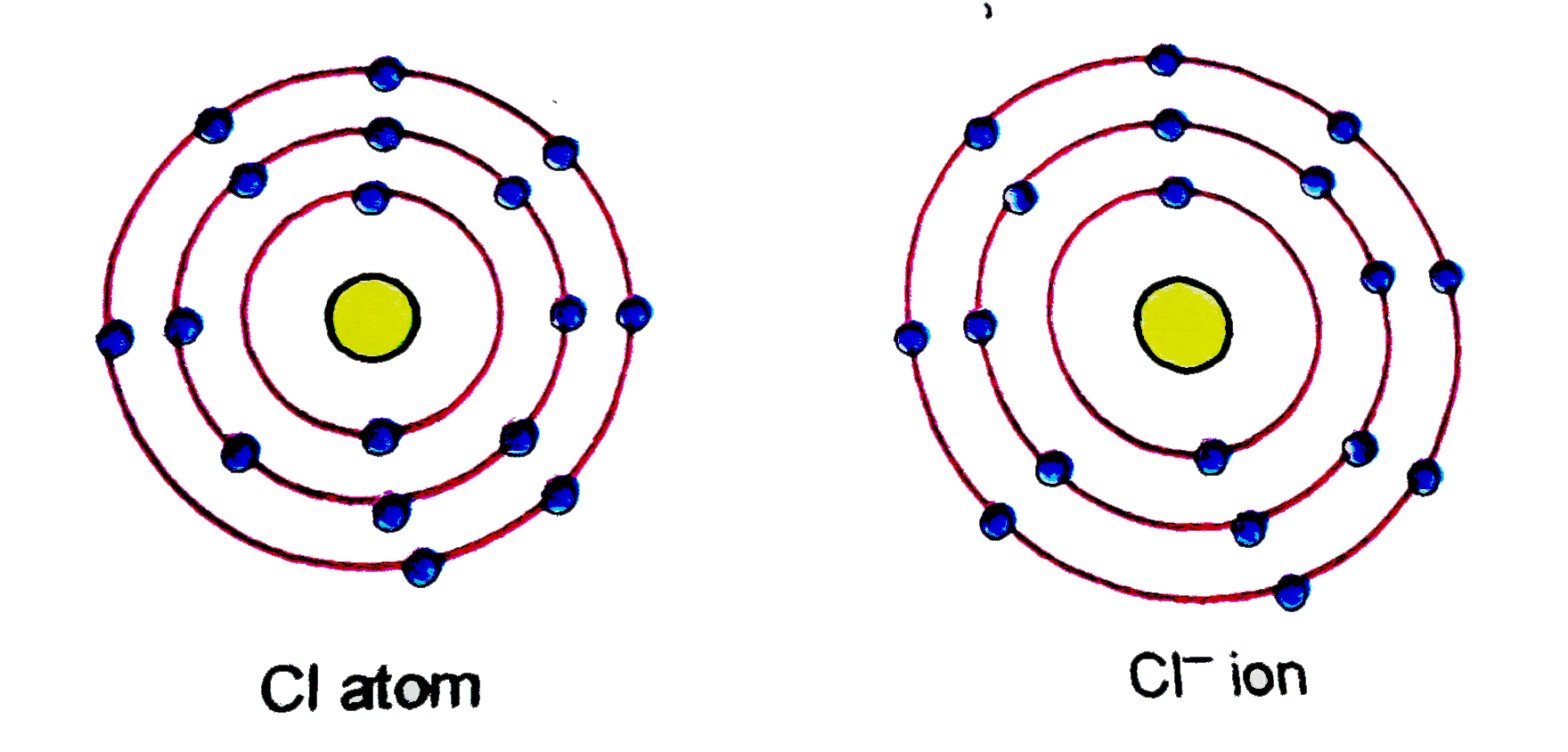
Chlorine Ion Radius . Its outermost electrons are alredy on the third energy level. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Although neither atoms nor ions have sharp. Radii of atoms and ions. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Welcome to the database of ionic radii. The following web interface allows listing and comparison of ionic and crystal radii with. When the chloride anion is formed, an electron is being added to that same. These radii are generally not the same (figure 8.2.2d 8.2.
from www.doubtnut.com
The following web interface allows listing and comparison of ionic and crystal radii with. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Welcome to the database of ionic radii. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Its outermost electrons are alredy on the third energy level. Radii of atoms and ions. These radii are generally not the same (figure 8.2.2d 8.2. When the chloride anion is formed, an electron is being added to that same. Although neither atoms nor ions have sharp.
(a) Give the schematic atomic structures of chlorine atom and chloride
Chlorine Ion Radius Although neither atoms nor ions have sharp. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). Radii of atoms and ions. This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Its outermost electrons are alredy on the third energy level. When the chloride anion is formed, an electron is being added to that same. The following web interface allows listing and comparison of ionic and crystal radii with. Welcome to the database of ionic radii. These radii are generally not the same (figure 8.2.2d 8.2. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp.
From www.gauthmath.com
The atomic and ionic radii for sodium and chlorine are shown in the Chlorine Ion Radius An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). This page explains the various measures of atomic radius, and then looks at. Chlorine Ion Radius.
From www.shutterstock.com
Types Atomic Radius Chemical Element Atomic Stock Vector (Royalty Free Chlorine Ion Radius When the chloride anion is formed, an electron is being added to that same. The following web interface allows listing and comparison of ionic and crystal radii with. Radii of atoms and ions. 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. This page explains the various measures of atomic radius,. Chlorine Ion Radius.
From sciencenotes.org
Atomic Radius and Ionic Radius Chlorine Ion Radius Radii of atoms and ions. Although neither atoms nor ions have sharp. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. These radii are generally not the same (figure 8.2.2d. Chlorine Ion Radius.
From sciencenotes.org
Chlorine Facts Chlorine Ion Radius Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. When the chloride anion is formed, an electron is being added to that same. 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp. These radii are generally not. Chlorine Ion Radius.
From chem.libretexts.org
2.8 Sizes of Atoms and Ions Chemistry LibreTexts Chlorine Ion Radius Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Although neither atoms nor ions have sharp. When the chloride anion is formed, an electron is being added to that same. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and. Chlorine Ion Radius.
From brainly.in
draw atomic structure of chlorine Brainly.in Chlorine Ion Radius Its outermost electrons are alredy on the third energy level. When the chloride anion is formed, an electron is being added to that same. 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. These radii are generally not the same (figure 8.2.2d 8.2. Although neither atoms nor ions have sharp. Conclusion,. Chlorine Ion Radius.
From sciencenotes.org
Atomic Radius and Ionic Radius Chlorine Ion Radius An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). The following web interface allows listing and comparison of ionic and crystal radii. Chlorine Ion Radius.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Ion Radius This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. When the chloride anion is formed, an electron is being added to that same. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der. Chlorine Ion Radius.
From animalia-life.club
Atomic Radius Diagram Chlorine Ion Radius Radii of atoms and ions. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. When the chloride anion is formed, an electron is being added to that same. The following web interface allows listing and comparison of ionic and crystal radii with. This page explains the. Chlorine Ion Radius.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Ion Radius Radii of atoms and ions. Although neither atoms nor ions have sharp. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Welcome to the database of ionic radii. These radii are generally not the same (figure 8.2.2d 8.2. The following web interface allows listing and comparison of ionic and crystal radii with. An. Chlorine Ion Radius.
From favpng.com
Electron Configuration Atomic Orbital Chlorine Chemistry, PNG Chlorine Ion Radius Radii of atoms and ions. Although neither atoms nor ions have sharp. Welcome to the database of ionic radii. Its outermost electrons are alredy on the third energy level. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between. Chlorine Ion Radius.
From www.shutterstock.com
32 Ionic radius 이미지, 스톡 사진 및 벡터 Shutterstock Chlorine Ion Radius This page explains the various measures of atomic radius, and then looks at the way it varies around the periodic. Welcome to the database of ionic radii. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Conclusion, chloride ion has a bigger atomic radius due to. Chlorine Ion Radius.
From brokeasshome.com
Periodic Table Of Elements Sorted By Atomic Radius Chlorine Ion Radius These radii are generally not the same (figure 8.2.2d 8.2. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). The following web. Chlorine Ion Radius.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Ion Radius The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Radii of atoms and ions. Welcome to the database of ionic radii. When the chloride anion is formed, an electron is being added to that same. This page explains the various measures of atomic radius, and then. Chlorine Ion Radius.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Ion Radius The following web interface allows listing and comparison of ionic and crystal radii with. Its outermost electrons are alredy on the third energy level. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +). Chlorine Ion Radius.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Chlorine Ion Radius The following web interface allows listing and comparison of ionic and crystal radii with. Although neither atoms nor ions have sharp. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for. Chlorine Ion Radius.
From chemistrylearnwithsangam.blogspot.com
chemistry knowledge Comparison between Covalent and Ionic Bond Chlorine Ion Radius An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). The following web interface allows listing and comparison of ionic and crystal radii. Chlorine Ion Radius.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Ion Radius Welcome to the database of ionic radii. 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. These radii are generally not the same (figure 8.2.2d 8.2. Its outermost electrons are alredy on the third energy level. An atom such as chlorine has both a covalent radius (the distance between the two. Chlorine Ion Radius.
From www.shutterstock.com
585 Chlorine electrons 이미지, 스톡 사진 및 벡터 Shutterstock Chlorine Ion Radius These radii are generally not the same (figure 8.2.2d 8.2. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Welcome to the database of ionic radii. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. The following web interface. Chlorine Ion Radius.
From www.savemyexams.com
Variations in Atomic & Ionic Radius CIE AS Chemistry Revision Notes 2025 Chlorine Ion Radius Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. When the chloride anion is formed, an electron is being added to that same. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Although neither atoms nor ions have sharp.. Chlorine Ion Radius.
From chemistrytalk.org
Ionic Radius Trends ChemTalk Chlorine Ion Radius Radii of atoms and ions. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for. Chlorine Ion Radius.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Chlorine Ion Radius Its outermost electrons are alredy on the third energy level. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. The following web interface allows listing and comparison of ionic and crystal radii with. 100 rows ionic radius, r ion, is the radius of a monatomic ion. Chlorine Ion Radius.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Ion Radius Its outermost electrons are alredy on the third energy level. The following web interface allows listing and comparison of ionic and crystal radii with. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. 100 rows ionic radius, r ion, is the radius of a monatomic ion. Chlorine Ion Radius.
From chem.libretexts.org
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic Chlorine Ion Radius Although neither atoms nor ions have sharp. Its outermost electrons are alredy on the third energy level. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. When the chloride anion is formed, an electron is being added to that same. Radii of atoms and ions. The following web interface allows listing and comparison. Chlorine Ion Radius.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Ion Radius These radii are generally not the same (figure 8.2.2d 8.2. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. The following web interface allows listing and comparison of ionic and. Chlorine Ion Radius.
From neetlab.com
Ionic Radius NEET Lab Chlorine Ion Radius An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). The atomic radius of the sodium atom (na) is 190 pm, and the. Chlorine Ion Radius.
From www.pathwaystochemistry.com
Atomic and Ionic Radii Pathways to Chemistry Chlorine Ion Radius When the chloride anion is formed, an electron is being added to that same. 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius. Chlorine Ion Radius.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Ion Radius Welcome to the database of ionic radii. Its outermost electrons are alredy on the third energy level. The following web interface allows listing and comparison of ionic and crystal radii with. These radii are generally not the same (figure 8.2.2d 8.2. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. An atom such. Chlorine Ion Radius.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Ion Radius When the chloride anion is formed, an electron is being added to that same. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Welcome to the database of ionic radii. Radii of atoms and ions. This page explains the various measures of atomic radius, and then. Chlorine Ion Radius.
From brainly.in
Draw the atomic structure of a chlorine ion Brainly.in Chlorine Ion Radius 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. When the chloride anion is formed, an electron is being added to that same. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. This page explains the various. Chlorine Ion Radius.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Chlorine Ion Radius When the chloride anion is formed, an electron is being added to that same. The atomic radius of the sodium atom (na) is 190 pm, and the ionic radius of sodium ion (na +) is 116 pm. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons. Although neither atoms nor ions have sharp.. Chlorine Ion Radius.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Chlorine Ion Radius An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). These radii are generally not the same (figure 8.2.2d 8.2. 100 rows ionic. Chlorine Ion Radius.
From chemistry.stackexchange.com
What is the atomic radius of chlorine? Chemistry Stack Exchange Chlorine Ion Radius 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl. Chlorine Ion Radius.
From gia-kharmon.blogspot.com
What Is the Atomic Radius of Chlorine Chlorine Ion Radius Its outermost electrons are alredy on the third energy level. Although neither atoms nor ions have sharp. The following web interface allows listing and comparison of ionic and crystal radii with. Welcome to the database of ionic radii. These radii are generally not the same (figure 8.2.2d 8.2. Conclusion, chloride ion has a bigger atomic radius due to an increase. Chlorine Ion Radius.
From courses.lumenlearning.com
Ionic Radius Introduction to Chemistry Chlorine Ion Radius The following web interface allows listing and comparison of ionic and crystal radii with. Its outermost electrons are alredy on the third energy level. Welcome to the database of ionic radii. 100 rows ionic radius, r ion, is the radius of a monatomic ion in an ionic crystal structure. These radii are generally not the same (figure 8.2.2d 8.2. Conclusion,. Chlorine Ion Radius.