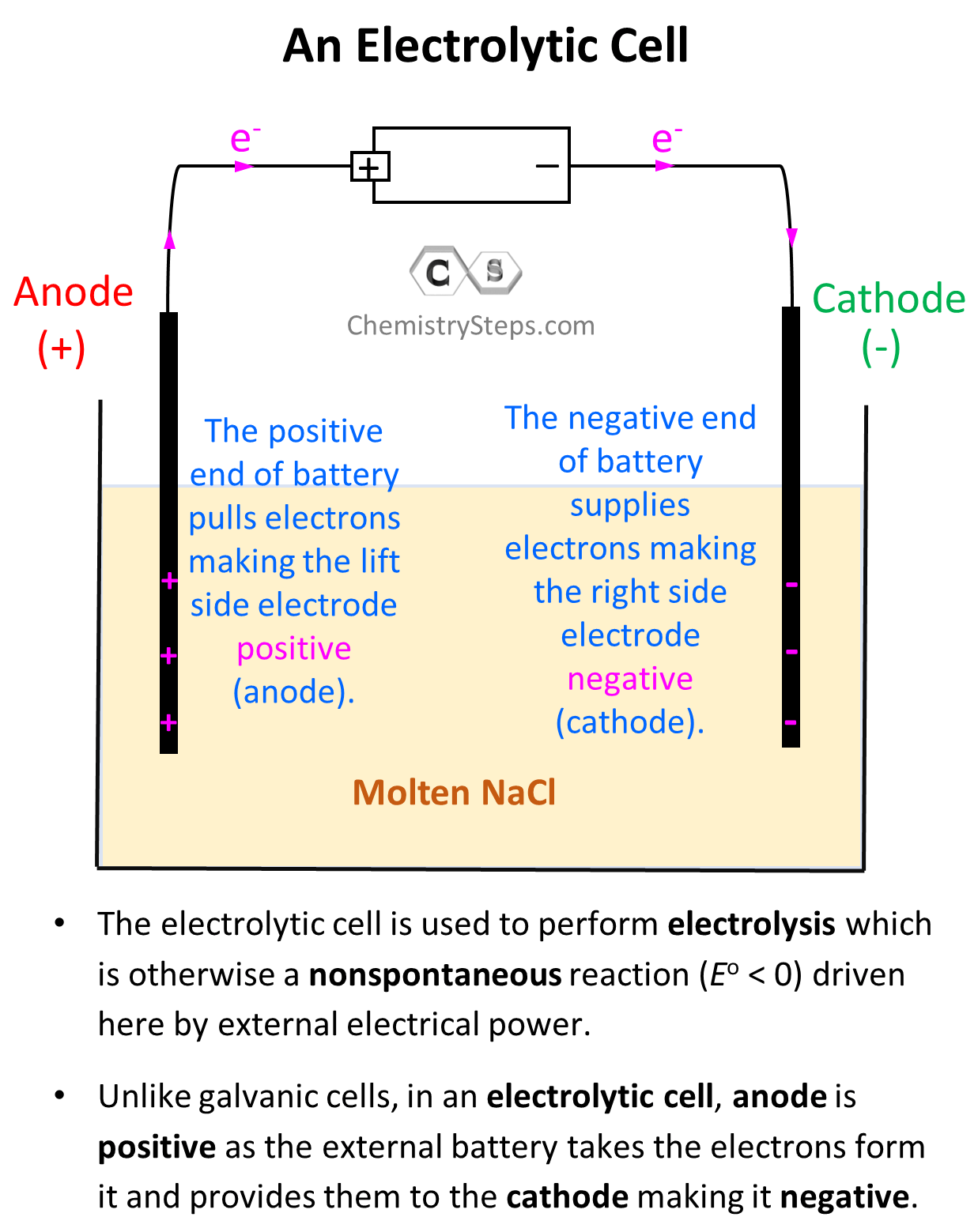
Electrolytic Cell In Everyday Life . Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Electrolytic cells use an electric current to drive a. Such a cell typically consists of two. Electroplating results in a thin. They charge many electronic devices, such as phones and electric cars. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; An important use for electrolytic cells is in electroplating. Electrolytic cells are crucial to functions of daily life; Only a single compartment is employed in most applications.
from general.chemistrysteps.com
Such a cell typically consists of two. Electrolytic cells use an electric current to drive a. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; They charge many electronic devices, such as phones and electric cars. An important use for electrolytic cells is in electroplating. Only a single compartment is employed in most applications. Electroplating results in a thin. Electrolytic cells are crucial to functions of daily life; Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa.
Electrolysis Chemistry Steps
Electrolytic Cell In Everyday Life In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Electroplating results in a thin. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. An important use for electrolytic cells is in electroplating. Electrolytic cells use an electric current to drive a. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Such a cell typically consists of two. Electrolytic cells are crucial to functions of daily life; Only a single compartment is employed in most applications. They charge many electronic devices, such as phones and electric cars.
From www.thoughtco.com
Electrochemical Cell Definition Electrolytic Cell In Everyday Life Only a single compartment is employed in most applications. They charge many electronic devices, such as phones and electric cars. An important use for electrolytic cells is in electroplating. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Electroplating results in a thin. Electrolytic cell, any device in. Electrolytic Cell In Everyday Life.
From www.youtube.com
Electrolytic Cells Nonspontaneous Redox Reactions YouTube Electrolytic Cell In Everyday Life Electrolytic cells are crucial to functions of daily life; In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt. Electrolytic Cell In Everyday Life.
From app.pandai.org
Electrolytic Cell Electrolytic Cell In Everyday Life Such a cell typically consists of two. They charge many electronic devices, such as phones and electric cars. Only a single compartment is employed in most applications. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential. Electrolytic Cell In Everyday Life.
From www.slideserve.com
PPT Electrolytic Cell PowerPoint Presentation, free download ID2809508 Electrolytic Cell In Everyday Life Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Electrolytic cells are crucial to functions of daily life; Such a cell typically consists of two. Electroplating results in a thin. Electrolytic cells. Electrolytic Cell In Everyday Life.
From mydigitalkemistry.com
What is Electrolytic Cell in Simple Words? Electrolytic Cell In Everyday Life Such a cell typically consists of two. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Only a single compartment is employed in most applications. Electroplating results in a thin. In this explainer, we will learn how to. Electrolytic Cell In Everyday Life.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrolytic Cell In Everyday Life Only a single compartment is employed in most applications. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Electrolytic cells use an electric current to drive a. In an electrolytic cell (right),. Electrolytic Cell In Everyday Life.
From slidetodoc.com
Electrolytic Cells Lab Lesson 10 Electrolytic cell Lab Electrolytic Cell In Everyday Life In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Electrolytic cells use an electric current to drive a. Such a cell typically consists of two. They charge many electronic devices, such as phones and electric cars. Electrolytic cell,. Electrolytic Cell In Everyday Life.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrolytic Cell In Everyday Life Such a cell typically consists of two. Electrolytic cells use an electric current to drive a. Electrolytic cells are crucial to functions of daily life; In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. They charge many electronic devices, such as phones and electric cars. In an electrolytic. Electrolytic Cell In Everyday Life.
From www.youtube.com
Uses of Electrochemistry in our daily life (Electrochemistry part 2 for Electrolytic Cell In Everyday Life An important use for electrolytic cells is in electroplating. Electroplating results in a thin. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Only a single compartment is employed in most applications. Electrolytic cell, any device in which. Electrolytic Cell In Everyday Life.
From www.nanoscience.com
Electrochemistry Nanoscience Instruments Electrolytic Cell In Everyday Life An important use for electrolytic cells is in electroplating. Such a cell typically consists of two. Electroplating results in a thin. Only a single compartment is employed in most applications. Electrolytic cells are crucial to functions of daily life; Electrolytic cells use an electric current to drive a. Electrolytic cell, any device in which electrical energy is converted to chemical. Electrolytic Cell In Everyday Life.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples Electrolytic Cell In Everyday Life They charge many electronic devices, such as phones and electric cars. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. An important use for electrolytic cells is in electroplating. Electrolytic cells are crucial to functions of daily life; Electrolytic cells use an electric current to drive a. Only. Electrolytic Cell In Everyday Life.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5570772 Electrolytic Cell In Everyday Life In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Such a cell typically consists of two. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Electroplating. Electrolytic Cell In Everyday Life.
From pandai.me
Electrolytic Cell Electrolytic Cell In Everyday Life An important use for electrolytic cells is in electroplating. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Electroplating results in a. Electrolytic Cell In Everyday Life.
From www.youtube.com
Electrolytic Cells YouTube Electrolytic Cell In Everyday Life An important use for electrolytic cells is in electroplating. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Such a cell typically. Electrolytic Cell In Everyday Life.
From chemistrypage.in
Electrolysis Definition, Principle and Electrolytic Cell Electrolytic Cell In Everyday Life In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; They charge many electronic devices, such as phones and electric cars. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. An important. Electrolytic Cell In Everyday Life.
From www.dreamstime.com
Electrolytic Cell Infographic Diagram with Components Stock Vector Electrolytic Cell In Everyday Life An important use for electrolytic cells is in electroplating. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Electrolytic cells are crucial to functions of daily life; Electroplating results in a thin. Only a single compartment is employed. Electrolytic Cell In Everyday Life.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Electrolytic Cell In Everyday Life In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. They charge many electronic devices, such as phones and electric cars. Such a cell typically consists of two. Electrolytic cells are crucial to functions of daily life; Electrolytic cell, any device in which electrical energy is converted to chemical. Electrolytic Cell In Everyday Life.
From www.pinterest.com
Electrolytic cell Electrolysis of NaCl Cell definition, Chemistry Electrolytic Cell In Everyday Life Electroplating results in a thin. Electrolytic cells use an electric current to drive a. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Such a cell typically consists of two. Electrolytic cells. Electrolytic Cell In Everyday Life.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Electrolytic Cell In Everyday Life Only a single compartment is employed in most applications. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Electroplating results in a thin. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox. Electrolytic Cell In Everyday Life.
From www.researchgate.net
Diagram of electrolytic cell. Download Scientific Diagram Electrolytic Cell In Everyday Life Electrolytic cells use an electric current to drive a. Electroplating results in a thin. Such a cell typically consists of two. Only a single compartment is employed in most applications. An important use for electrolytic cells is in electroplating. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes. Electrolytic Cell In Everyday Life.
From www.slideserve.com
PPT The Electrolytic Cell PowerPoint Presentation, free download ID Electrolytic Cell In Everyday Life Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Electrolytic cells are crucial to functions of daily life; Such a cell typically consists of two. They charge many electronic devices, such as phones and electric cars. Electrolytic cells use an electric current to drive a. Electroplating results in a thin. An important use. Electrolytic Cell In Everyday Life.
From www.researchgate.net
A simple secondary LeadAcid electrolytic cell with a liquid Electrolytic Cell In Everyday Life Electrolytic cells are crucial to functions of daily life; Such a cell typically consists of two. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Only a single compartment is employed in. Electrolytic Cell In Everyday Life.
From facts.net
16 Mindblowing Facts About Electrolytic Cell Electrolytic Cell In Everyday Life Only a single compartment is employed in most applications. Electrolytic cells are crucial to functions of daily life; Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Electrolytic cells use an electric current to drive a. Electroplating results in a thin. Such a cell typically consists of two. In an electrolytic cell (right),. Electrolytic Cell In Everyday Life.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Electrolytic Cell In Everyday Life They charge many electronic devices, such as phones and electric cars. An important use for electrolytic cells is in electroplating. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Such a cell typically consists of two. Electrolytic cell,. Electrolytic Cell In Everyday Life.
From www.researchgate.net
Schematic diagram of electrolytic cell Download Scientific Diagram Electrolytic Cell In Everyday Life Such a cell typically consists of two. Only a single compartment is employed in most applications. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Electrolytic cell, any device in which electrical energy is converted to chemical energy,. Electrolytic Cell In Everyday Life.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Electrolytic Cell In Everyday Life Electrolytic cells use an electric current to drive a. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; They charge many electronic devices, such as phones and electric cars. Electrolytic cell, any device in which electrical energy is. Electrolytic Cell In Everyday Life.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya Electrolytic Cell In Everyday Life Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Electroplating results in a thin. Only a single compartment is employed in most applications. Electrolytic cells use an electric current to drive a. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions.. Electrolytic Cell In Everyday Life.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Electrolytic Cell In Everyday Life Electrolytic cells are crucial to functions of daily life; Electrolytic cells use an electric current to drive a. Only a single compartment is employed in most applications. Such a cell typically consists of two. An important use for electrolytic cells is in electroplating. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential. Electrolytic Cell In Everyday Life.
From www.pinterest.co.kr
What is Electrolytic cell Science chemistry, Electrochemical cell Electrolytic Cell In Everyday Life Such a cell typically consists of two. Electrolytic cells are crucial to functions of daily life; An important use for electrolytic cells is in electroplating. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; In this explainer, we. Electrolytic Cell In Everyday Life.
From www.vecteezy.com
Electrolysis of electrolyte solution in electrochemistry vector Electrolytic Cell In Everyday Life In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Electrolytic cells are crucial to functions of daily life; In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox. Electrolytic Cell In Everyday Life.
From dokumen.tips
(PPT) Electrochemical & Electrolytic Cells Using Redox Reactions in Electrolytic Cell In Everyday Life Electrolytic cells are crucial to functions of daily life; They charge many electronic devices, such as phones and electric cars. Electroplating results in a thin. Electrolytic cells use an electric current to drive a. An important use for electrolytic cells is in electroplating. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten. Electrolytic Cell In Everyday Life.
From thechemistrynotes.com
Electrolytic Cell Definition, Principle, Components, Applications Electrolytic Cell In Everyday Life In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Electroplating results in a thin. They charge many electronic devices, such as phones and electric cars. In this explainer, we will learn how to describe conditions and applications for. Electrolytic Cell In Everyday Life.
From proper-cooking.info
Cell Real Life Electrolytic Cell In Everyday Life Electrolytic cells use an electric current to drive a. Only a single compartment is employed in most applications. In this explainer, we will learn how to describe conditions and applications for the electrolysis of molten salts and salt solutions. Electroplating results in a thin. In an electrolytic cell (right), an external source of electrical energy is used to generate a. Electrolytic Cell In Everyday Life.
From www.slideserve.com
PPT The Electrolytic Cell PowerPoint Presentation, free download ID Electrolytic Cell In Everyday Life Electrolytic cells are crucial to functions of daily life; In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Only a single compartment. Electrolytic Cell In Everyday Life.
From www.savemyexams.com
Electrolytic Cells SL IB Chemistry Revision Notes 2025 Electrolytic Cell In Everyday Life Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. An important use for electrolytic cells is in electroplating. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; Such a cell typically. Electrolytic Cell In Everyday Life.