Temperature Of Nitrogen Gas . Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Data from nist standard reference database 69: Top, gas phase heat capacity (shomate equation), references. The temperature of liquid nitrogen is −195.79 °c (77 k; Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Liquid nitrogen is very cold! Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly.
from www.chemix-chemistry-software.com
Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Liquid nitrogen is very cold! Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Data from nist standard reference database 69: Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. The temperature of liquid nitrogen is −195.79 °c (77 k; Top, gas phase heat capacity (shomate equation), references. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind.
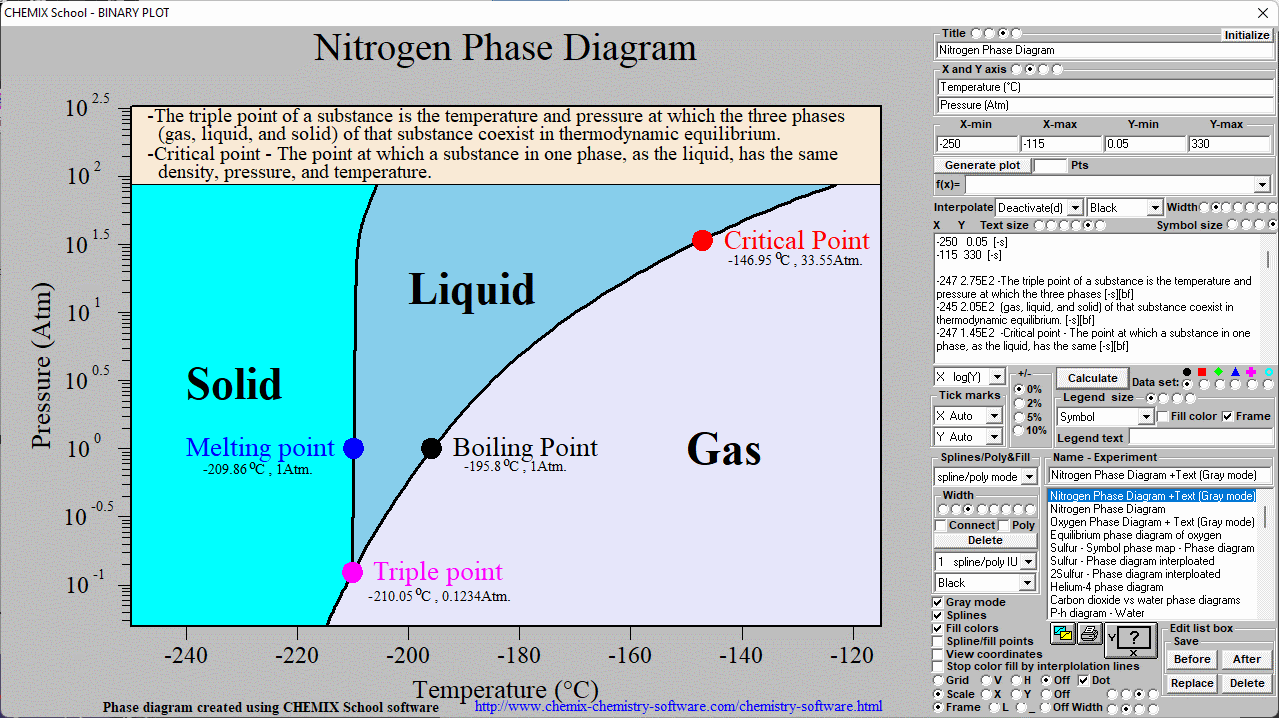
Nitrogen phase diagram
Temperature Of Nitrogen Gas Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Liquid nitrogen is very cold! Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Data from nist standard reference database 69: The temperature of liquid nitrogen is −195.79 °c (77 k; Top, gas phase heat capacity (shomate equation), references. At room temperature and pressure, liquid nitrogen boils into nitrogen gas.
From pressbooks.nscc.ca
7.8 Real Gases Introductory Chemistry 1st Canadian / NSCC Edition Temperature Of Nitrogen Gas Liquid nitrogen is very cold! The temperature of liquid nitrogen is −195.79 °c (77 k; Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of. Temperature Of Nitrogen Gas.
From www.researchgate.net
Rotational temperature of nitrogen gas evaluated from the emission Temperature Of Nitrogen Gas The temperature of liquid nitrogen is −195.79 °c (77 k; At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and. Temperature Of Nitrogen Gas.
From www.alamy.com
Nitrogen is the chemical element. At room temperature, it is a gas of Temperature Of Nitrogen Gas At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Top, gas phase heat capacity (shomate equation), references. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Liquid. Temperature Of Nitrogen Gas.
From www.myxxgirl.com
Figure A Phase Diagram P T Of Nitrogen The Normal Melting My XXX Hot Girl Temperature Of Nitrogen Gas Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Top, gas phase heat capacity (shomate equation), references. Data from nist standard reference database 69: The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that. Temperature Of Nitrogen Gas.
From www.haikudeck.com
Nitrogen by Sandra Bilku Temperature Of Nitrogen Gas Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Top, gas phase heat capacity (shomate equation), references. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air. Temperature Of Nitrogen Gas.
From www.youtube.com
Explain Joule Thomson effect with Nitrogen TS diagram YouTube Temperature Of Nitrogen Gas Data from nist standard reference database 69: Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The temperature of liquid nitrogen is −195.79 °c (77 k; Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Liquid nitrogen is very cold! Nitrogen can also be produced on a large scale by. Temperature Of Nitrogen Gas.
From www.bartleby.com
Answered An initial mixture of nitrogen gas and… bartleby Temperature Of Nitrogen Gas Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Liquid nitrogen is very cold! The temperature of liquid. Temperature Of Nitrogen Gas.
From www.chemix-chemistry-software.com
Nitrogen phase diagram Temperature Of Nitrogen Gas Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting. Temperature Of Nitrogen Gas.
From www.researchgate.net
Nitrogen T/S diagram Download Scientific Diagram Temperature Of Nitrogen Gas The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Data from nist standard reference database 69: Top, gas phase heat capacity (shomate equation), references. The temperature of liquid nitrogen is −195.79 °c (77. Temperature Of Nitrogen Gas.
From www.chegg.com
Solved For many purposes we can treat nitrogen (N2) as an Temperature Of Nitrogen Gas The temperature of liquid nitrogen is −195.79 °c (77 k; Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Data from nist standard reference database 69: Liquid nitrogen is very. Temperature Of Nitrogen Gas.
From galvinconanstuart.blogspot.com
Nitrogen Phase Diagram Pressure Temperature General Wiring Diagram Temperature Of Nitrogen Gas Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Liquid nitrogen is very cold! Top, gas phase heat capacity (shomate equation), references. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting. Temperature Of Nitrogen Gas.
From www.numerade.com
SOLVED Calculate the density of nitrogen gas at a temperature of 101 Temperature Of Nitrogen Gas The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Data from nist standard reference database 69: At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Liquid nitrogen is very cold! The. Temperature Of Nitrogen Gas.
From www.toppr.com
A quantity of 56g of nitrogen gas is enclosed in a rigid vessel at a Temperature Of Nitrogen Gas Data from nist standard reference database 69: Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons. Temperature Of Nitrogen Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Temperature Of Nitrogen Gas Temperature Of Nitrogen Gas The temperature of liquid nitrogen is −195.79 °c (77 k; The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Nitrogen can also be produced on a large scale by. Temperature Of Nitrogen Gas.
From chem.libretexts.org
7.2 The Gas Laws Chemistry LibreTexts Temperature Of Nitrogen Gas The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Top, gas phase heat capacity (shomate equation), references. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Liquid. Temperature Of Nitrogen Gas.
From www.toppr.com
r.m.s velocity of nitrogen molecules at NTP is Temperature Of Nitrogen Gas At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Top, gas phase heat capacity (shomate equation), references. The temperature of liquid nitrogen is −195.79 °c (77 k; The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Data from nist standard reference database 69:. Temperature Of Nitrogen Gas.
From www.chegg.com
Solved An initial mixture of nitrogen gas and hydrogen gas Temperature Of Nitrogen Gas At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Top, gas phase heat capacity (shomate equation), references. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. The temperature of liquid nitrogen is −195.79 °c (77 k; Liquid nitrogen has. Temperature Of Nitrogen Gas.
From www.chegg.com
Solved Estimate the average speed of Nitrogen gas molecule Temperature Of Nitrogen Gas Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Liquid nitrogen is very cold! The temperature of liquid nitrogen is −195.79 °c (77 k; The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that. Temperature Of Nitrogen Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Temperature Of Nitrogen Gas Temperature Of Nitrogen Gas The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Liquid nitrogen is very cold! Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in. Temperature Of Nitrogen Gas.
From www.coursehero.com
[Solved] Find the specific heat at constant pressure of nitrogen gas Temperature Of Nitrogen Gas Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Top, gas phase heat capacity (shomate equation), references. Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Liquid nitrogen is very cold! Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the. Temperature Of Nitrogen Gas.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Temperature Of Nitrogen Gas Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Top, gas phase heat capacity (shomate equation), references. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Calculation of thermodynamic state variables of nitrogen in. Temperature Of Nitrogen Gas.
From www.chegg.com
The equation of state of gaseous nitrogen at low Temperature Of Nitrogen Gas Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. The temperature of liquid nitrogen is −195.79 °c (77 k; Liquid nitrogen is very cold! Calculation. Temperature Of Nitrogen Gas.
From www.researchgate.net
Temperature distribution of nitrogen precooling. Download Scientific Temperature Of Nitrogen Gas Data from nist standard reference database 69: The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Liquid nitrogen is very cold! Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water. Temperature Of Nitrogen Gas.
From www.chegg.com
Solved Nitrogen gas flows through a compressor and a heat Temperature Of Nitrogen Gas Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly.. Temperature Of Nitrogen Gas.
From pressbooks.bccampus.ca
3.2 Real gas and compressibility factor Introduction to Engineering Temperature Of Nitrogen Gas At room temperature and pressure, liquid nitrogen boils into nitrogen gas. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Data from nist standard reference database 69: Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Nitrogen is a colourless, odourless gas,. Temperature Of Nitrogen Gas.
From www.researchgate.net
Density of nitrogen with change in temperature and at different Temperature Of Nitrogen Gas Top, gas phase heat capacity (shomate equation), references. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind.. Temperature Of Nitrogen Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Dew Point Of Nitrogen Gas Temperature Of Nitrogen Gas Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Top, gas phase heat capacity (shomate equation), references. Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Liquid nitrogen has many uses, but poses risks. Temperature Of Nitrogen Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Enthalpy Of Formation Temperature Of Nitrogen Gas Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Top, gas phase heat capacity (shomate equation), references. Data from nist standard reference database 69: Nitrogen can also be produced on. Temperature Of Nitrogen Gas.
From www.numerade.com
SOLVED The figure shows a pV diagram for 8.3 g of ideal nitrogen gas Temperature Of Nitrogen Gas Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. The temperature of liquid nitrogen is −195.79 °c (77 k; Top, gas phase heat capacity (shomate. Temperature Of Nitrogen Gas.
From www.bigstockphoto.com
Nitrogen Chemical Image & Photo (Free Trial) Bigstock Temperature Of Nitrogen Gas Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. Top, gas phase heat capacity (shomate equation), references. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. The boiling temperature of nitrogen is −195.8 °c. Temperature Of Nitrogen Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Heat Of Formation Temperature Of Nitrogen Gas Liquid nitrogen is very cold! Top, gas phase heat capacity (shomate equation), references. The temperature of liquid nitrogen is −195.79 °c (77 k; The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation. Temperature Of Nitrogen Gas.
From www.numerade.com
For many purposes, we can treat nitrogen (N2) as an ideal gas at Temperature Of Nitrogen Gas Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Top, gas phase heat. Temperature Of Nitrogen Gas.
From www.researchgate.net
Oxygen, nitrogen and argon vapor pressure curves. Download Scientific Temperature Of Nitrogen Gas Liquid nitrogen is very cold! Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Nitrogen can also. Temperature Of Nitrogen Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Heat Capacity Of Nitrogen Gas Temperature Of Nitrogen Gas Top, gas phase heat capacity (shomate equation), references. The temperature of liquid nitrogen is −195.79 °c (77 k; Liquid nitrogen is very cold! Data from nist standard reference database 69: Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. At room. Temperature Of Nitrogen Gas.
From mungfali.com
Nitrogen Pressure Temperature Chart Temperature Of Nitrogen Gas The boiling temperature of nitrogen is −195.8 °c (−320.4 °f), about 13 °c (−23 °f) below that of oxygen, which is therefore left behind. Nitrogen is a colourless, odourless gas, which condenses at −195.8 °c to a colourless, mobile liquid. Top, gas phase heat capacity (shomate equation), references. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and. Temperature Of Nitrogen Gas.