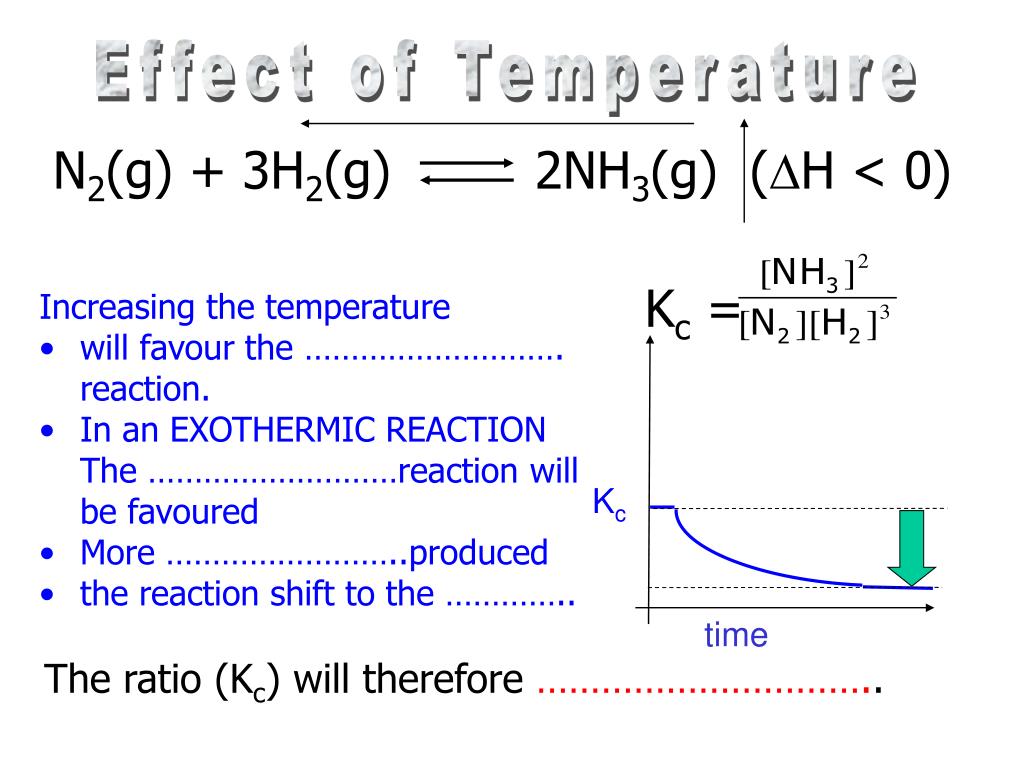
Why Does Kc Increase When Temperature Decreases . An increase in temperature will. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Increasing the temperature decreases the value of the equilibrium constant. Changes in temperature affect the value of the equilibrium constant, k c; For an endothermic reaction such as: In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. In the equilibrium above, decreasing the temperature will favour the backwards reaction. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. If δh is positive, reaction is endothermic, then: Solubility increases as temperature increases. A decrease in temperature favours the exothermic reaction. Where the forward reaction is endothermic, increasing the temperature increases the value of the. Ks increases with temperature meaning dissolving is.
from www.slideserve.com
Increasing the temperature decreases the value of the equilibrium constant. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. Changes in temperature affect the value of the equilibrium constant, k c; In the equilibrium above, decreasing the temperature will favour the backwards reaction. An increase in temperature will. Where the forward reaction is endothermic, increasing the temperature increases the value of the. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. A decrease in temperature favours the exothermic reaction. Ks increases with temperature meaning dissolving is. If δh is positive, reaction is endothermic, then:
PPT Chemical Equilibrium PowerPoint Presentation, free download ID
Why Does Kc Increase When Temperature Decreases A decrease in temperature favours the exothermic reaction. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. When temperature is the stress that affects a system at equilibrium, there are two important consequences: An increase in temperature will. Ks increases with temperature meaning dissolving is. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. A decrease in temperature favours the exothermic reaction. For an endothermic reaction such as: Increasing the temperature decreases the value of the equilibrium constant. Solubility increases as temperature increases. If δh is positive, reaction is endothermic, then: In the equilibrium above, decreasing the temperature will favour the backwards reaction. Where the forward reaction is endothermic, increasing the temperature increases the value of the. Changes in temperature affect the value of the equilibrium constant, k c;
From slideplayer.com
Chemical Equilibrium Chapter ppt download Why Does Kc Increase When Temperature Decreases If δh is positive, reaction is endothermic, then: An increase in temperature will. For an endothermic reaction such as: Solubility increases as temperature increases. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. When. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT Equilibrium and Stability Constants PowerPoint Presentation, free Why Does Kc Increase When Temperature Decreases Changes in temperature affect the value of the equilibrium constant, k c; A decrease in temperature favours the exothermic reaction. An increase in temperature will. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. If δh is positive, reaction is endothermic, then: Where the forward reaction is endothermic, increasing the temperature increases the value. Why Does Kc Increase When Temperature Decreases.
From www.numerade.com
SOLVEDWith increase in temperature, equilibrium constant of a reaction Why Does Kc Increase When Temperature Decreases In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. For an endothermic reaction such as: A decrease in temperature favours the exothermic reaction. If δh is positive, reaction is endothermic, then: Where the forward. Why Does Kc Increase When Temperature Decreases.
From www.numerade.com
SOLVED predict the change in the equilibrium constant KC that would Why Does Kc Increase When Temperature Decreases In the equilibrium above, decreasing the temperature will favour the backwards reaction. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. A decrease in temperature favours the exothermic reaction. For an endothermic reaction such as: Increasing the temperature decreases the value of the equilibrium constant. An increase in temperature will. If δh is positive,. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Why Does Kc Increase When Temperature Decreases If δh is positive, reaction is endothermic, then: Ks increases with temperature meaning dissolving is. Where the forward reaction is endothermic, increasing the temperature increases the value of the. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. Solubility increases as temperature increases. An increase in temperature will. In the equilibrium above, decreasing the. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT IC S2 K3 The only factor that affects the value of Kc is Why Does Kc Increase When Temperature Decreases In the equilibrium above, decreasing the temperature will favour the backwards reaction. Where the forward reaction is endothermic, increasing the temperature increases the value of the. If δh is positive, reaction is endothermic, then: When temperature is the stress that affects a system at equilibrium, there are two important consequences: Solubility increases as temperature increases. Increasing the temperature decreases the. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Why Does Kc Increase When Temperature Decreases In the equilibrium above, decreasing the temperature will favour the backwards reaction. Increasing the temperature decreases the value of the equilibrium constant. For an endothermic reaction such as: When temperature is the stress that affects a system at equilibrium, there are two important consequences: Changes in temperature affect the value of the equilibrium constant, k c; Solubility increases as temperature. Why Does Kc Increase When Temperature Decreases.
From www.researchgate.net
Deviations of (A) Kc and (B) βvKn {\beta}_v Kn on the temperature Why Does Kc Increase When Temperature Decreases An increase in temperature will. Solubility increases as temperature increases. Increasing the temperature decreases the value of the equilibrium constant. If δh is positive, reaction is endothermic, then: In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. Changes in temperature affect the value of the equilibrium constant, k c;. Why Does Kc Increase When Temperature Decreases.
From tfvp.org
Why Is Kc Affected By Temperature Unveiling The Thermodynamic Mystery Why Does Kc Increase When Temperature Decreases For an endothermic reaction such as: Increasing the temperature decreases the value of the equilibrium constant. If δh is positive, reaction is endothermic, then: A decrease in temperature favours the exothermic reaction. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Changes in temperature affect the value of the equilibrium constant, k c;. Why Does Kc Increase When Temperature Decreases.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Why Does Kc Increase When Temperature Decreases In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. If δh is positive, reaction is endothermic, then: A decrease in temperature favours the exothermic reaction. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Where the forward reaction is endothermic, increasing the. Why Does Kc Increase When Temperature Decreases.
From slideplayer.com
Chemical Equilibrium PART ppt download Why Does Kc Increase When Temperature Decreases Where the forward reaction is endothermic, increasing the temperature increases the value of the. In the equilibrium above, decreasing the temperature will favour the backwards reaction. If δh is positive, reaction is endothermic, then: Increasing the temperature decreases the value of the equilibrium constant. Ks increases with temperature meaning dissolving is. Solubility increases as temperature increases. An increase in temperature. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT Factors that Affect Equilibrium PowerPoint Presentation ID3270711 Why Does Kc Increase When Temperature Decreases If δh is positive, reaction is endothermic, then: A decrease in temperature favours the exothermic reaction. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Where the forward reaction is endothermic, increasing the temperature increases the value of the. An increase in temperature will. Solubility increases as temperature increases. For an endothermic reaction. Why Does Kc Increase When Temperature Decreases.
From www.numerade.com
SOLVEDNumber of Question 2 particles with 4) In an endotheraiccnerc Why Does Kc Increase When Temperature Decreases Solubility increases as temperature increases. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. In the equilibrium above, decreasing the temperature will favour the backwards reaction. If δh is positive, reaction is endothermic, then: When temperature is the stress that affects a system at equilibrium, there are two important consequences: Increasing the temperature decreases. Why Does Kc Increase When Temperature Decreases.
From slidetodoc.com
Chemical Equilibrium Chapter 14 Chemical Equilibrium 14 1 Why Does Kc Increase When Temperature Decreases When temperature is the stress that affects a system at equilibrium, there are two important consequences: Ks increases with temperature meaning dissolving is. Increasing the temperature decreases the value of the equilibrium constant. An increase in temperature will. Changes in temperature affect the value of the equilibrium constant, k c; If δh is positive, reaction is endothermic, then: In the. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Why Does Kc Increase When Temperature Decreases When temperature is the stress that affects a system at equilibrium, there are two important consequences: For an endothermic reaction such as: A decrease in temperature favours the exothermic reaction. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. Increasing the temperature decreases the value of the equilibrium constant.. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT Reaction Equilibrium in Ideal Gas Mixture PowerPoint Presentation Why Does Kc Increase When Temperature Decreases Ks increases with temperature meaning dissolving is. Increasing the temperature decreases the value of the equilibrium constant. Where the forward reaction is endothermic, increasing the temperature increases the value of the. For an endothermic reaction such as: In the equilibrium above, decreasing the temperature will favour the backwards reaction. If δh is positive, reaction is endothermic, then: When temperature is. Why Does Kc Increase When Temperature Decreases.
From worksheetmediastanley.z21.web.core.windows.net
Equilibrium Constant Kc Formula Why Does Kc Increase When Temperature Decreases Ks increases with temperature meaning dissolving is. In the equilibrium above, decreasing the temperature will favour the backwards reaction. Increasing the temperature decreases the value of the equilibrium constant. For an endothermic reaction such as: Changes in temperature affect the value of the equilibrium constant, k c; The value of the equilibrium constant, k, for a given reaction is dependent. Why Does Kc Increase When Temperature Decreases.
From slideplayer.com
Chapter 15 Chemical Equilibrium John A. Schreifels Chemistry ppt download Why Does Kc Increase When Temperature Decreases When temperature is the stress that affects a system at equilibrium, there are two important consequences: Ks increases with temperature meaning dissolving is. A decrease in temperature favours the exothermic reaction. For an endothermic reaction such as: In the equilibrium above, decreasing the temperature will favour the backwards reaction. If δh is positive, reaction is endothermic, then: An increase in. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT Chapter 9 Solutions PowerPoint Presentation, free download ID Why Does Kc Increase When Temperature Decreases A decrease in temperature favours the exothermic reaction. For an endothermic reaction such as: In the equilibrium above, decreasing the temperature will favour the backwards reaction. Solubility increases as temperature increases. If δh is positive, reaction is endothermic, then: Changes in temperature affect the value of the equilibrium constant, k c; Ks increases with temperature meaning dissolving is. In the. Why Does Kc Increase When Temperature Decreases.
From slideplayer.com
Chemistry NCEA L2 2.6 Chemical Reactivity. ppt download Why Does Kc Increase When Temperature Decreases An increase in temperature will. Increasing the temperature decreases the value of the equilibrium constant. A decrease in temperature favours the exothermic reaction. Ks increases with temperature meaning dissolving is. In the equilibrium above, decreasing the temperature will favour the backwards reaction. Changes in temperature affect the value of the equilibrium constant, k c; The value of the equilibrium constant,. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Why Does Kc Increase When Temperature Decreases For an endothermic reaction such as: Ks increases with temperature meaning dissolving is. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. Increasing the temperature decreases the value of the equilibrium constant. An increase in temperature will. In the equilibrium above, decreasing the temperature will favour the backwards reaction. Changes in temperature affect the. Why Does Kc Increase When Temperature Decreases.
From slideplayer.com
What is Density? is the amount of matter in a specific volume. ppt Why Does Kc Increase When Temperature Decreases Solubility increases as temperature increases. Increasing the temperature decreases the value of the equilibrium constant. Where the forward reaction is endothermic, increasing the temperature increases the value of the. In the equilibrium above, decreasing the temperature will favour the backwards reaction. A decrease in temperature favours the exothermic reaction. If δh is positive, reaction is endothermic, then: In the case. Why Does Kc Increase When Temperature Decreases.
From www.youtube.com
Effect of Change in Temperature on Equilibrium Constant (Kc) in Pashto Why Does Kc Increase When Temperature Decreases Changes in temperature affect the value of the equilibrium constant, k c; For an endothermic reaction such as: If δh is positive, reaction is endothermic, then: The value of the equilibrium constant, k, for a given reaction is dependent on temperature. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on. Why Does Kc Increase When Temperature Decreases.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Why Does Kc Increase When Temperature Decreases Solubility increases as temperature increases. Changes in temperature affect the value of the equilibrium constant, k c; The value of the equilibrium constant, k, for a given reaction is dependent on temperature. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. An increase in temperature will. For an endothermic. Why Does Kc Increase When Temperature Decreases.
From www.numerade.com
SOLVED At a given temperature, the equilibrium constant Kc for the Why Does Kc Increase When Temperature Decreases A decrease in temperature favours the exothermic reaction. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. Changes in temperature affect the value of the equilibrium constant, k c; When temperature is the stress that affects a system at equilibrium, there are two important consequences: Ks increases with temperature. Why Does Kc Increase When Temperature Decreases.
From www.researchgate.net
Correlation between temperature and Kc Download Scientific Diagram Why Does Kc Increase When Temperature Decreases If δh is positive, reaction is endothermic, then: Changes in temperature affect the value of the equilibrium constant, k c; For an endothermic reaction such as: The value of the equilibrium constant, k, for a given reaction is dependent on temperature. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on. Why Does Kc Increase When Temperature Decreases.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Why Does Kc Increase When Temperature Decreases When temperature is the stress that affects a system at equilibrium, there are two important consequences: Ks increases with temperature meaning dissolving is. For an endothermic reaction such as: The value of the equilibrium constant, k, for a given reaction is dependent on temperature. An increase in temperature will. If δh is positive, reaction is endothermic, then: A decrease in. Why Does Kc Increase When Temperature Decreases.
From www.youtube.com
19 Only temperature affects Kc YouTube Why Does Kc Increase When Temperature Decreases Ks increases with temperature meaning dissolving is. A decrease in temperature favours the exothermic reaction. In the equilibrium above, decreasing the temperature will favour the backwards reaction. For an endothermic reaction such as: Increasing the temperature decreases the value of the equilibrium constant. If δh is positive, reaction is endothermic, then: When temperature is the stress that affects a system. Why Does Kc Increase When Temperature Decreases.
From brainly.in
why does solubility of gases in liquids decreases with increase in Why Does Kc Increase When Temperature Decreases If δh is positive, reaction is endothermic, then: In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. Solubility increases as temperature increases. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Ks increases with temperature meaning dissolving is. For an endothermic reaction. Why Does Kc Increase When Temperature Decreases.
From www.youtube.com
16.2 Effect of temperature on the rate constant k (HL) YouTube Why Does Kc Increase When Temperature Decreases Where the forward reaction is endothermic, increasing the temperature increases the value of the. In the equilibrium above, decreasing the temperature will favour the backwards reaction. If δh is positive, reaction is endothermic, then: An increase in temperature will. When temperature is the stress that affects a system at equilibrium, there are two important consequences: The value of the equilibrium. Why Does Kc Increase When Temperature Decreases.
From www.youtube.com
Effect of Temperature on Equilibrium Constants (Extension Material Why Does Kc Increase When Temperature Decreases The value of the equilibrium constant, k, for a given reaction is dependent on temperature. When temperature is the stress that affects a system at equilibrium, there are two important consequences: An increase in temperature will. Increasing the temperature decreases the value of the equilibrium constant. Changes in temperature affect the value of the equilibrium constant, k c; Ks increases. Why Does Kc Increase When Temperature Decreases.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Why Does Kc Increase When Temperature Decreases If δh is positive, reaction is endothermic, then: The value of the equilibrium constant, k, for a given reaction is dependent on temperature. In the equilibrium above, decreasing the temperature will favour the backwards reaction. Where the forward reaction is endothermic, increasing the temperature increases the value of the. In the case of temperature, the value of the equilibrium has. Why Does Kc Increase When Temperature Decreases.
From www.w3schools.blog
Factors affecting the rate of a reaction Temperature W3schools Why Does Kc Increase When Temperature Decreases In the equilibrium above, decreasing the temperature will favour the backwards reaction. A decrease in temperature favours the exothermic reaction. Increasing the temperature decreases the value of the equilibrium constant. Solubility increases as temperature increases. Ks increases with temperature meaning dissolving is. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. When temperature is. Why Does Kc Increase When Temperature Decreases.
From www.youtube.com
Why temperature decreases with altitude?why_temperature_decreases_with Why Does Kc Increase When Temperature Decreases Increasing the temperature decreases the value of the equilibrium constant. In the equilibrium above, decreasing the temperature will favour the backwards reaction. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. A decrease in temperature favours the exothermic reaction. Ks increases with temperature meaning dissolving is. If δh is positive, reaction is endothermic, then:. Why Does Kc Increase When Temperature Decreases.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Why Does Kc Increase When Temperature Decreases If δh is positive, reaction is endothermic, then: For an endothermic reaction such as: When temperature is the stress that affects a system at equilibrium, there are two important consequences: Changes in temperature affect the value of the equilibrium constant, k c; Increasing the temperature decreases the value of the equilibrium constant. Where the forward reaction is endothermic, increasing the. Why Does Kc Increase When Temperature Decreases.