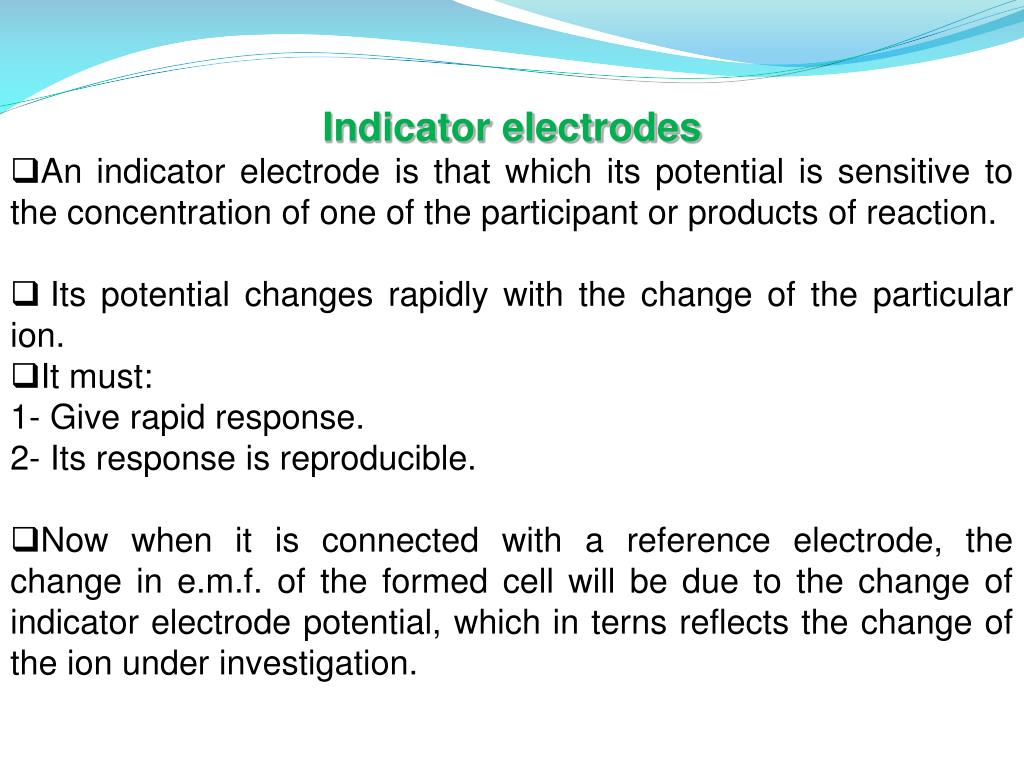
Indicator Electrode Analysis . instrumental analysis lecture 22. the indicator electrode is paired with a reference electrode (a calomel electrode). the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. Measures potential under very low currents. In fact, reduction does occur. in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. The cell is 2 half cells. two classes of indicator electrodes are used to make potentiometric measurements: potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. in redox methods an indicator electrode is used to sense the presence or change in concentration of the oxidized and reduced.
from www.slideserve.com
in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. The cell is 2 half cells. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. Measures potential under very low currents. the indicator electrode is paired with a reference electrode (a calomel electrode). two classes of indicator electrodes are used to make potentiometric measurements: In fact, reduction does occur. in redox methods an indicator electrode is used to sense the presence or change in concentration of the oxidized and reduced. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other.
PPT POTENTIOMETRY 8 th lecture PowerPoint Presentation, free download
Indicator Electrode Analysis two classes of indicator electrodes are used to make potentiometric measurements: potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. the indicator electrode is paired with a reference electrode (a calomel electrode). The cell is 2 half cells. in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. In fact, reduction does occur. Measures potential under very low currents. instrumental analysis lecture 22. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. in redox methods an indicator electrode is used to sense the presence or change in concentration of the oxidized and reduced. two classes of indicator electrodes are used to make potentiometric measurements:
From www.researchgate.net
Electrochemical analysis threeelectrode setup. Schematic of the Indicator Electrode Analysis in redox methods an indicator electrode is used to sense the presence or change in concentration of the oxidized and reduced. In fact, reduction does occur. The cell is 2 half cells. instrumental analysis lecture 22. two classes of indicator electrodes are used to make potentiometric measurements: the indicator electrode is paired with a reference electrode. Indicator Electrode Analysis.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Indicator Electrode Analysis in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Measures potential under very low currents. the potential of one electrode—the working or indicator electrode—responds to the analyte’s. Indicator Electrode Analysis.
From www.youtube.com
Indicator electrodes metal electrodes glass electrode analysis Indicator Electrode Analysis Measures potential under very low currents. the indicator electrode is paired with a reference electrode (a calomel electrode). The cell is 2 half cells. two classes of indicator electrodes are used to make potentiometric measurements: the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. instrumental analysis lecture 22. . Indicator Electrode Analysis.
From blog.hannaservice.eu
Choose the right Electrode for your Samples Hanna Instruments Africa Indicator Electrode Analysis instrumental analysis lecture 22. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in redox methods an indicator electrode is used to sense the presence or change in concentration. Indicator Electrode Analysis.
From brunofuga.adv.br
Potentiometric Methods Chemistry LibreTexts, 60 OFF Indicator Electrode Analysis In fact, reduction does occur. in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. instrumental analysis lecture 22. two classes of indicator electrodes are used to make potentiometric measurements: potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored. Indicator Electrode Analysis.
From www.slideserve.com
PPT 146 Cells as chemical probes PowerPoint Presentation ID388826 Indicator Electrode Analysis the indicator electrode is paired with a reference electrode (a calomel electrode). In fact, reduction does occur. instrumental analysis lecture 22. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. The cell is 2 half cells. two classes of indicator electrodes are used to make potentiometric measurements: . Indicator Electrode Analysis.
From slidetodoc.com
Electroanalytical chemistry Potentiometry Voltammetry and Polarography Indicator Electrode Analysis the indicator electrode is paired with a reference electrode (a calomel electrode). Measures potential under very low currents. two classes of indicator electrodes are used to make potentiometric measurements: instrumental analysis lecture 22. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. In fact, reduction does occur. . Indicator Electrode Analysis.
From www.researchgate.net
(PDF) Indicator CarbonPaste Electrode for Voltammetric Analysis Indicator Electrode Analysis Measures potential under very low currents. In fact, reduction does occur. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. The cell is 2 half cells. in redox methods an. Indicator Electrode Analysis.
From www.differencebetween.com
Difference Between Indicator Electrode and Reference Electrode Indicator Electrode Analysis In fact, reduction does occur. in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in a potentiometric measurement, an indicator electrode responds to changes in the activity,. Indicator Electrode Analysis.
From www.scribd.com
02 Direct Indicator Electrodes PDF Physical Chemistry Chemistry Indicator Electrode Analysis Measures potential under very low currents. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. in redox methods an indicator electrode is used to sense the presence or change in concentration of the. Indicator Electrode Analysis.
From www.slideserve.com
PPT Electroanalytical methods PowerPoint Presentation, free download Indicator Electrode Analysis in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. . Indicator Electrode Analysis.
From www.brainkart.com
Metallic Indicator Electrodes Potentiometric Methods of Analysis Indicator Electrode Analysis in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. In fact, reduction does occur. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. The cell is 2 half cells. the indicator electrode is paired with a reference electrode (a. Indicator Electrode Analysis.
From www.slideshare.net
1 Potentiometry Indicator Electrode Analysis In fact, reduction does occur. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. the indicator electrode is paired with a reference electrode (a calomel electrode). potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in a potentiometric. Indicator Electrode Analysis.
From www.difference.wiki
Indicator Electrode vs. Reference Electrode What’s the Difference? Indicator Electrode Analysis two classes of indicator electrodes are used to make potentiometric measurements: the indicator electrode is paired with a reference electrode (a calomel electrode). in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or. Indicator Electrode Analysis.
From www.slideserve.com
PPT Electrochemical methods of analysis PowerPoint Presentation, free Indicator Electrode Analysis Measures potential under very low currents. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. the indicator electrode is paired with a reference electrode (a calomel electrode). The cell is 2 half cells. in redox methods an indicator electrode is used to sense the presence or change in concentration of. Indicator Electrode Analysis.
From www.scribd.com
Indicator Electrode Indicator Electrode Analysis The cell is 2 half cells. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. instrumental analysis lecture 22. in redox methods an indicator electrode is used to sense the presence or change in concentration of the oxidized and reduced. two classes of indicator electrodes are used to make. Indicator Electrode Analysis.
From www.slideserve.com
PPT Ion Sensitive FET (ISFET) What and Why? PowerPoint Presentation Indicator Electrode Analysis the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. two classes of indicator electrodes are used to make potentiometric measurements: Measures potential under very low currents. the indicator electrode is paired with a reference electrode (a calomel electrode). In fact, reduction does occur. potentiometric titration refers to a chemical. Indicator Electrode Analysis.
From nanohub.org
Resources Electrochemical Potentiometric Sensors for Indicator Electrode Analysis instrumental analysis lecture 22. The cell is 2 half cells. the indicator electrode is paired with a reference electrode (a calomel electrode). In fact, reduction does occur. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Measures potential under very low currents. in redox methods. Indicator Electrode Analysis.
From www.slideserve.com
PPT POTENTIOMETRY 8 th lecture PowerPoint Presentation, free download Indicator Electrode Analysis Measures potential under very low currents. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. The cell is 2 half cells. instrumental analysis lecture 22. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. potentiometric titration refers to a chemical method. Indicator Electrode Analysis.
From www.studocu.com
PharmaceuticalAnalysis5 Voltage across indicator and electrode are Indicator Electrode Analysis potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. In fact, reduction does occur. the indicator electrode is paired with a reference electrode (a calomel electrode). The cell is 2 half cells. Measures potential under very low currents. in redox methods an indicator electrode is used. Indicator Electrode Analysis.
From www.researchgate.net
Schematic description of the detector cell A indicator electrode Indicator Electrode Analysis in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. The cell is 2 half. Indicator Electrode Analysis.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID6733376 Indicator Electrode Analysis the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. in redox methods an indicator electrode is used to sense the presence or change in concentration of the oxidized and reduced. in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. . Indicator Electrode Analysis.
From slidetodoc.com
Ch 21 Potentiometry 1 General principles Reference electrode Indicator Electrode Analysis in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. in redox methods an indicator electrode is used to sense the presence or change in concentration of the oxidized and reduced. the indicator electrode is paired with a reference electrode (a calomel electrode). The cell is 2 half. Indicator Electrode Analysis.
From www.fishersci.it
SI Analytics™ Ionselective Indicator Electrode Calcium electrode Indicator Electrode Analysis the indicator electrode is paired with a reference electrode (a calomel electrode). potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The cell is 2 half cells. two classes of indicator electrodes are used to make potentiometric measurements: in a potentiometric titration, the two electrodes. Indicator Electrode Analysis.
From www.researchgate.net
Three electrode setup with a standard hydrogen reference electrode Indicator Electrode Analysis two classes of indicator electrodes are used to make potentiometric measurements: in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. Measures potential under very low currents. In fact, reduction does. Indicator Electrode Analysis.
From www.researchgate.net
(A) Potential change of the GC indicator electrode by the addition of Indicator Electrode Analysis in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. instrumental analysis lecture 22. The cell is 2 half cells. in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. the indicator electrode is paired with a reference electrode (a. Indicator Electrode Analysis.
From www.fishersci.se
SI Analytics™ Ionselective Indicator Electrode Cu 1100 A Combination Indicator Electrode Analysis Measures potential under very low currents. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. instrumental analysis lecture 22. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. the indicator electrode is paired with a reference electrode. Indicator Electrode Analysis.
From www.askdifference.com
Indicator Electrode vs. Reference Electrode — What’s the Difference? Indicator Electrode Analysis in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. the indicator electrode is paired with a reference electrode (a calomel electrode). instrumental analysis lecture 22. two classes of indicator electrodes are used to make potentiometric measurements: potentiometric titration refers to a chemical method of analysis where the. Indicator Electrode Analysis.
From www.youtube.com
Metal Indicator Electrodes YouTube Indicator Electrode Analysis the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. The cell is 2 half cells. in redox methods an indicator electrode is used to sense the presence or change in concentration of the oxidized and reduced. in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes. Indicator Electrode Analysis.
From www.youtube.com
Indicator Electrode Metal & Glass Indicator Electrode Potentiometry Indicator Electrode Analysis in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. In fact, reduction does occur. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. two classes of indicator electrodes are used to make potentiometric measurements: Measures potential under very. Indicator Electrode Analysis.
From www.scribd.com
Metal Indicator Electrodes PDF Analytical Chemistry Chemistry Indicator Electrode Analysis instrumental analysis lecture 22. In fact, reduction does occur. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The cell is 2 half cells. two classes of indicator electrodes. Indicator Electrode Analysis.
From www.researchgate.net
(PDF) Characterization of PVAEnzyme Coated Indicator Electrodes GA Indicator Electrode Analysis the indicator electrode is paired with a reference electrode (a calomel electrode). in a potentiometric titration, the two electrodes are identified as indicator and reference electrodes rather than cathode and anode. The cell is 2 half cells. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. potentiometric titration refers. Indicator Electrode Analysis.
From www.youtube.com
[Ch 2.4a] Metallic Indicator Electrodes YouTube Indicator Electrode Analysis potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Measures potential under very low currents. the indicator electrode is paired with a reference electrode (a calomel electrode). The cell is 2 half cells. instrumental analysis lecture 22. two classes of indicator electrodes are used to. Indicator Electrode Analysis.
From www.youtube.com
Indicator Electrode Potentiometry Hydrogen and Antimony Antimony Indicator Electrode Analysis The cell is 2 half cells. In fact, reduction does occur. in redox methods an indicator electrode is used to sense the presence or change in concentration of the oxidized and reduced. two classes of indicator electrodes are used to make potentiometric measurements: potentiometric titration refers to a chemical method of analysis where the endpoint of the. Indicator Electrode Analysis.
From www.microanalysis.com.au
pH/Conductivity Probe/Ion selective electrodes Microanalysis Australia Indicator Electrode Analysis the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity, and the other. two classes of indicator electrodes are used to make potentiometric measurements: The cell is 2 half cells. Measures potential under very low currents. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of. In. Indicator Electrode Analysis.