Calculation Of Z Effective . Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. Zeff = the effective nuclear. Calculating effective nuclear charge involves understanding the z and s values. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. For example, in lithium (li), none of the three electrons feel the full +3. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals.
from solvedlib.com
An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. Calculating effective nuclear charge involves understanding the z and s values. z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. For example, in lithium (li), none of the three electrons feel the full +3. Zeff = the effective nuclear.
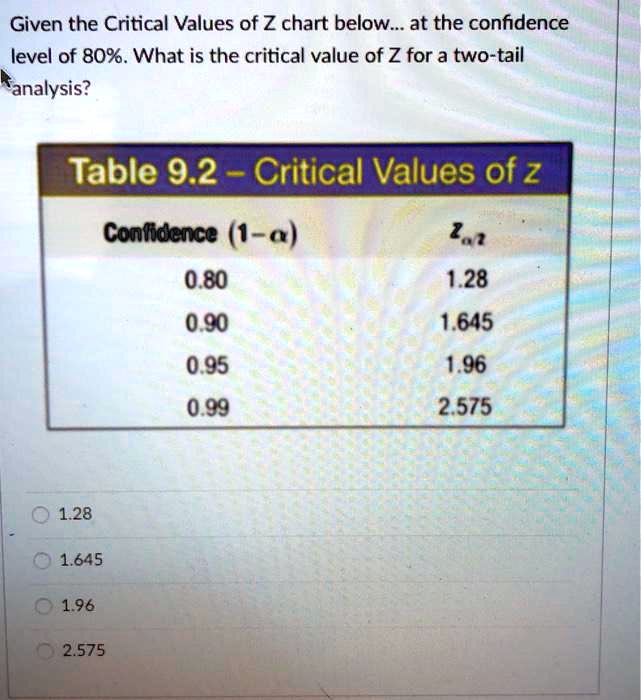
Given the Critical Values of Z chart below__at the c… SolvedLib
Calculation Of Z Effective Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). For example, in lithium (li), none of the three electrons feel the full +3. Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: Zeff = the effective nuclear. Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. Calculating effective nuclear charge involves understanding the z and s values.
From slidesharenow.blogspot.com
How To Calculate Z Effective slideshare Calculation Of Z Effective An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Besides, the formula for calculating the. Calculation Of Z Effective.
From slideplayer.com
doc. IEEE ppt download Calculation Of Z Effective Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Zeff = the effective nuclear. z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. Slater's rule states that the net charge experienced. Calculation Of Z Effective.
From edutized.com
Calculating The Probability Of Z Score Edutized Calculation Of Z Effective z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: An effective nuclear charge calculator is a tool used in chemistry to determine. Calculation Of Z Effective.
From www.educba.com
Z Score Formula Calculator (Examples with Excel template) Calculation Of Z Effective Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Zeff = the effective nuclear. Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. z is atomic number, and s. Calculation Of Z Effective.
From byjus.com
What is Z effective how to calculate it? Explain. Calculation Of Z Effective Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. For example, in lithium (li), none of the three electrons feel the full +3. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator.. Calculation Of Z Effective.
From www.youtube.com
calculation of z effective and screening constant YouTube Calculation Of Z Effective The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. Zeff = the effective nuclear. Calculating effective nuclear charge involves. Calculation Of Z Effective.
From www.breakingatom.com
Nuclear Charge Calculation Of Z Effective An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. Calculating effective nuclear charge involves understanding the z. Calculation Of Z Effective.
From slidesharenow.blogspot.com
How To Calculate Z Effective slideshare Calculation Of Z Effective For example, in lithium (li), none of the three electrons feel the full +3. Zeff = the effective nuclear. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. Slater's rule states that the net charge. Calculation Of Z Effective.
From slidesharenow.blogspot.com
How To Calculate Z Effective slideshare Calculation Of Z Effective z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other. Calculation Of Z Effective.
From byjus.com
14. If Zeffective of sodium is x then Z effective of carbon is a)x +.90 Calculation Of Z Effective Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: Calculating effective nuclear charge involves understanding the z and s values. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). For example, in lithium (li), none of the three electrons feel the full. Calculation Of Z Effective.
From mathsathome.com
How To Understand And Calculate ZScores Calculation Of Z Effective Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Zeff = the effective nuclear. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. z is atomic number, and. Calculation Of Z Effective.
From www.inchcalculator.com
ZScore Calculator (with Formulas & Steps) Inch Calculator Calculation Of Z Effective Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Learn how to calculate the effect of shielding on electrons. Calculation Of Z Effective.
From slidesharenow.blogspot.com
How To Calculate Z Effective slideshare Calculation Of Z Effective Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Learn how to calculate the effect. Calculation Of Z Effective.
From byjus.com
25. How to calculate effective nuclear charge (Z effective) of N+ Calculation Of Z Effective An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Zeff = the effective nuclear. Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. Besides, the formula for calculating. Calculation Of Z Effective.
From www.chegg.com
Solved 5. Using the table below of Zefi, calculate the first Calculation Of Z Effective The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. Slater's rule is used to estimate the effective nuclear charge experienced by an. Calculation Of Z Effective.
From slidesharenow.blogspot.com
How To Calculate Z Effective slideshare Calculation Of Z Effective Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. The amount of positive charge experienced by any individual electron. Calculation Of Z Effective.
From slidesharenow.blogspot.com
How To Calculate Z Effective slideshare Calculation Of Z Effective Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: Slater's rule is used to estimate the effective nuclear charge experienced by an electron. Calculation Of Z Effective.
From www.indianchemistry.com
Understanding z Effective Trends in the Periodic Table and Its Calculation Of Z Effective Zeff = the effective nuclear. Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: Calculating effective nuclear charge involves understanding the z and s values. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). An effective nuclear charge calculator is a tool. Calculation Of Z Effective.
From mathsathome.com
How To Understand And Calculate ZScores Calculation Of Z Effective The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. Calculating effective nuclear charge involves understanding. Calculation Of Z Effective.
From mathsux.org
How to Calculate ZScore? Statistics Math Lessons Calculation Of Z Effective An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Zeff = the effective nuclear. For example, in. Calculation Of Z Effective.
From eduinput.com
ZScoreDefinition, Calculation, Interpretation, and Examples Calculation Of Z Effective Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. For example, in lithium (li), none of the three electrons feel the full +3. Zeff = the effective nuclear. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. z . Calculation Of Z Effective.
From www.youtube.com
Effective Nuclear Charge (Zeffective) Chapter 7 Part 1 YouTube Calculation Of Z Effective Zeff = the effective nuclear. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number. Calculation Of Z Effective.
From slidesharenow.blogspot.com
How To Calculate Z Effective slideshare Calculation Of Z Effective Zeff = the effective nuclear. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. For example, in lithium (li), none of the three electrons feel the full +3. The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Calculating effective nuclear charge involves understanding the z. Calculation Of Z Effective.
From solvedlib.com
Given the Critical Values of Z chart below__at the c… SolvedLib Calculation Of Z Effective Zeff = the effective nuclear. Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. For example, in lithium (li), none of the three electrons feel the full +3. Slater's rule is used to. Calculation Of Z Effective.
From slidesharenow.blogspot.com
How To Calculate Z Effective slideshare Calculation Of Z Effective For example, in lithium (li), none of the three electrons feel the full +3. Zeff = the effective nuclear. Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. Besides, the formula for calculating the effective. Calculation Of Z Effective.
From www.youtube.com
The `Z_(eff)` for 3d electron of `Cr` `4s` electron of `Cr` `3d Calculation Of Z Effective Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Learn how to calculate the effect of shielding on electrons. Calculation Of Z Effective.
From www.qualitygurus.com
Two Sample Z Hypothesis Test Quality Gurus Calculation Of Z Effective Learn how to calculate the effect of shielding on electrons with our effective nuclear charge calculator. z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. Zeff = the effective nuclear. For example, in lithium (li), none of the. Calculation Of Z Effective.
From www.inchcalculator.com
ZScore Calculator (with Formulas & Steps) Inch Calculator Calculation Of Z Effective z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. For example, in lithium (li), none of the three electrons feel the full +3. Slater's rule states that the net charge experienced by an electron is equal to the. Calculation Of Z Effective.
From mathsux.org
How to Calculate ZScore? Statistics Math Lessons Calculation Of Z Effective Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. Besides, the formula for calculating the effective nuclear charge of a. Calculation Of Z Effective.
From thetoptutors.blogspot.com
How To Find Z Score With Standard Deviation And Mean Calculation Of Z Effective An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. For example, in lithium (li), none of the three electrons feel the full +3. Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: Calculating effective nuclear charge involves understanding the z . Calculation Of Z Effective.
From www.statology.org
How to Find ZScores Given Area (With Examples) Calculation Of Z Effective Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the. Calculation Of Z Effective.
From www.youtube.com
ZScores & Its Application Calculation and Formula of ZScore Calculation Of Z Effective Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. Zeff = the effective nuclear. Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. The amount of positive charge experienced by. Calculation Of Z Effective.
From www.wikihow.com
How to Calculate Z Scores 15 Steps (with Pictures) wikiHow Calculation Of Z Effective Slater's rule states that the net charge experienced by an electron is equal to the charge expected from a given number of protons minus the net charge from other electrons. Calculating effective nuclear charge involves understanding the z and s values. z is atomic number, and s requires the use of slater’s. Calculation Of Z Effective.
From www.researchgate.net
Zvalues for sample size calculation Download Scientific Diagram Calculation Of Z Effective The amount of positive charge experienced by any individual electron is the effective nuclear charge (\(z_{eff}\)). Besides, the formula for calculating the effective nuclear charge of a single electron is as follows: Slater's rule is used to estimate the effective nuclear charge experienced by an electron in different atomic orbitals. Zeff = the effective nuclear. z is atomic. Calculation Of Z Effective.
From www.youtube.com
How to Calculate Z Scores YouTube Calculation Of Z Effective For example, in lithium (li), none of the three electrons feel the full +3. z is atomic number, and s requires the use of slater’s rules to determine an electron cloud shielding value between the nucleus and the electron under consideration. Slater's rule states that the net charge experienced by an electron is equal to the. Calculation Of Z Effective.