Gas Laws Anesthesia . The carefully mixed gas is used to calibrate anesthetic gas monitors. By comparison of calculated and measured volumetric results it is shown that at atmospheric. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. The kinetic theory of gases makes the following assumptions: Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. There are several gas laws that apply to human physiology. It begins by using boyle's law to calculate the volume of oxygen.
from www.studocu.com
Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. By comparison of calculated and measured volumetric results it is shown that at atmospheric. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. It begins by using boyle's law to calculate the volume of oxygen. There are several gas laws that apply to human physiology. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. The kinetic theory of gases makes the following assumptions: The carefully mixed gas is used to calibrate anesthetic gas monitors. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia.
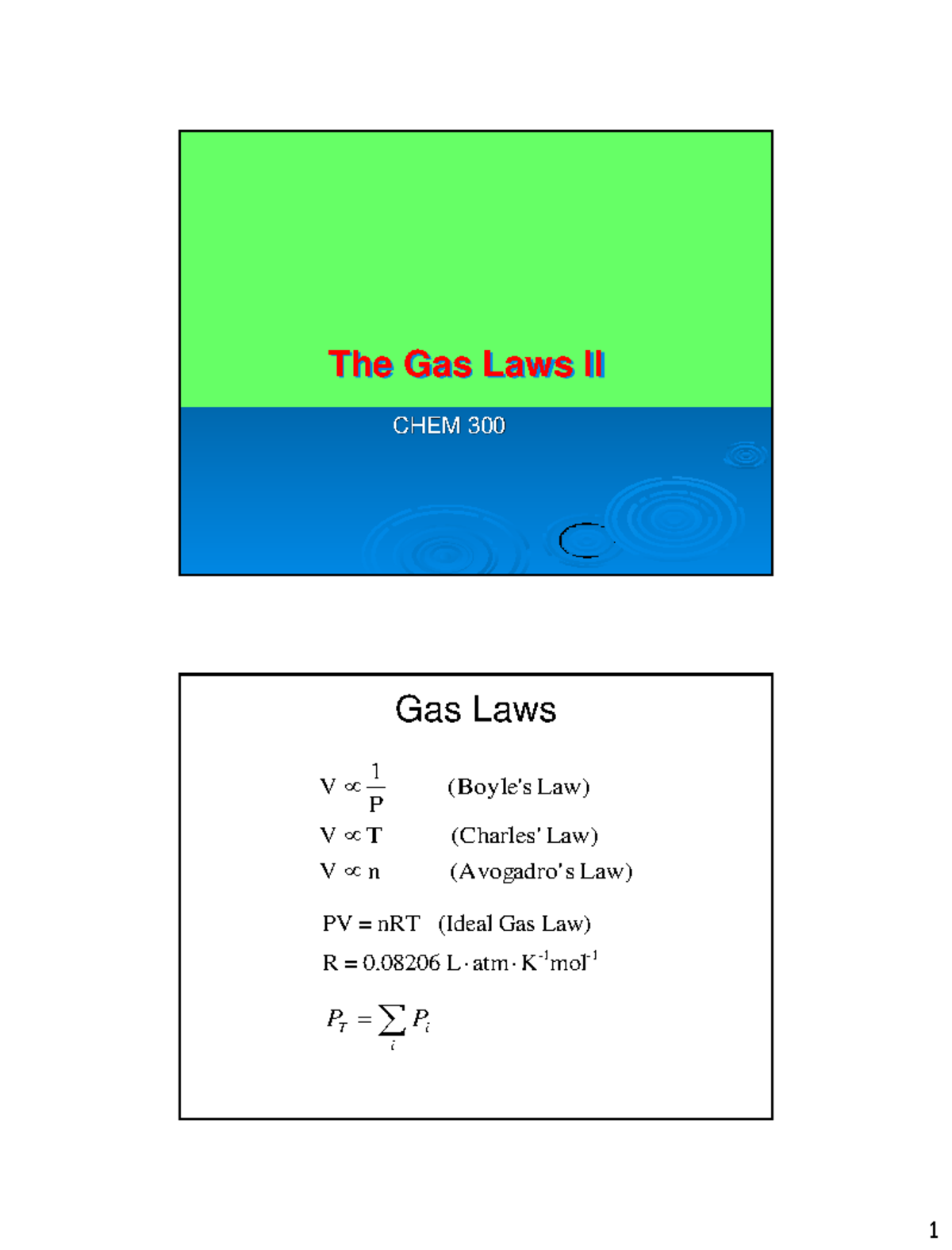
Lecture 3 Gas Laws part 2 CHEM 300 The Gas Laws II õ i PP iT Law) s
Gas Laws Anesthesia There are several gas laws that apply to human physiology. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. The carefully mixed gas is used to calibrate anesthetic gas monitors. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. It begins by using boyle's law to calculate the volume of oxygen. The kinetic theory of gases makes the following assumptions: The document discusses various gas laws and their applications in anesthesia and respiratory physiology. By comparison of calculated and measured volumetric results it is shown that at atmospheric. There are several gas laws that apply to human physiology.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Gas Laws Anesthesia Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. By comparison of calculated and measured volumetric results it is shown that at atmospheric. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. There. Gas Laws Anesthesia.
From www.youtube.com
Gas laws and Anesthesia saneeshpj YouTube Gas Laws Anesthesia There are several gas laws that apply to human physiology. It begins by using boyle's law to calculate the volume of oxygen. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. By comparison of calculated and measured volumetric results it is shown that at atmospheric. The kinetic theory. Gas Laws Anesthesia.
From www.youtube.com
Gas Laws Shorts gaslaws anesthesia anesthesiologist Gas Laws Anesthesia By comparison of calculated and measured volumetric results it is shown that at atmospheric. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. It begins by using boyle's law to calculate. Gas Laws Anesthesia.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Gas Laws Anesthesia It begins by using boyle's law to calculate the volume of oxygen. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. Accurate measurements and understanding relationships. Gas Laws Anesthesia.
From facts.net
19 Mindblowing Facts About Gas Laws Gas Laws Anesthesia There are several gas laws that apply to human physiology. It begins by using boyle's law to calculate the volume of oxygen. By comparison of calculated and measured volumetric results it is shown that at atmospheric. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. The carefully mixed gas is used to calibrate anesthetic gas monitors. Accurate measurements and understanding relationships like. Gas Laws Anesthesia.
From in.pinterest.com
This sheet gives the formulas for the Gas Laws. MA.912.HSAAPR.C Gas Laws Anesthesia Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. The kinetic theory of gases makes the following assumptions: By comparison of calculated and measured volumetric results it is shown that at atmospheric. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is. Gas Laws Anesthesia.
From www.studocu.com
Gases 3 Gases HW [Q] Explain the three gas laws in terms of Gas Laws Anesthesia The kinetic theory of gases makes the following assumptions: The carefully mixed gas is used to calibrate anesthetic gas monitors. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. By comparison of calculated and measured volumetric results it is shown that at atmospheric. It begins by using boyle's. Gas Laws Anesthesia.
From browsegrades.net
Exploring the Gas Laws Browsegrades Gas Laws Anesthesia The document discusses various gas laws and their applications in anesthesia and respiratory physiology. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. There are several gas laws that apply to human physiology. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. The carefully mixed gas is used to calibrate anesthetic gas monitors. The kinetic. Gas Laws Anesthesia.
From www.scribd.com
Gas Laws Cheat Sheet PDF Gas Laws Anesthesia The carefully mixed gas is used to calibrate anesthetic gas monitors. By comparison of calculated and measured volumetric results it is shown that at atmospheric. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. There are several gas laws that apply to human physiology. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. The kinetic. Gas Laws Anesthesia.
From mungfali.com
Gas Laws Formula Sheet Gas Laws Anesthesia By comparison of calculated and measured volumetric results it is shown that at atmospheric. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. The carefully mixed gas is used to calibrate anesthetic gas monitors. The kinetic theory of gases makes the following assumptions: Universal gas constant (r) the universal gas constant (also known. Gas Laws Anesthesia.
From aneskey.com
Chapter 30 Starling’s Law and Cardiac Dysfunction Anesthesia Key Gas Laws Anesthesia It begins by using boyle's law to calculate the volume of oxygen. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. By comparison of calculated and measured volumetric results it is shown that at atmospheric. The document discusses various gas laws and their applications in anesthesia. Gas Laws Anesthesia.
From www.studocu.com
Gas laws Dr Description and explanation of gas laws to help with Gas Laws Anesthesia The document discusses various gas laws and their applications in anesthesia and respiratory physiology. By comparison of calculated and measured volumetric results it is shown that at atmospheric. The carefully mixed gas is used to calibrate anesthetic gas monitors. The kinetic theory of gases makes the following assumptions: Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. Accurate measurements and understanding relationships. Gas Laws Anesthesia.
From www.scribd.com
Gas Law PDF Gases Pressure Gas Laws Anesthesia There are several gas laws that apply to human physiology. The kinetic theory of gases makes the following assumptions: Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. It begins by using boyle's law to calculate the volume of oxygen. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. The carefully mixed gas is used to calibrate. Gas Laws Anesthesia.
From www.worksheetsplanet.com
The Gas Laws Gas Laws Anesthesia Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. There are several gas laws that apply to human physiology. The carefully mixed gas is used to calibrate anesthetic gas monitors. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. By. Gas Laws Anesthesia.
From www.scribd.com
Gas Law Brochure PDF Gas Laws Anesthesia It begins by using boyle's law to calculate the volume of oxygen. The kinetic theory of gases makes the following assumptions: There are several gas laws that apply to human physiology. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the.. Gas Laws Anesthesia.
From www.studocu.com
Lecture 3 Gas Laws part 2 CHEM 300 The Gas Laws II õ i PP iT Law) s Gas Laws Anesthesia By comparison of calculated and measured volumetric results it is shown that at atmospheric. The carefully mixed gas is used to calibrate anesthetic gas monitors. The kinetic theory of gases makes the following assumptions: Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. It begins by. Gas Laws Anesthesia.
From www.slideshare.net
gas laws in anesthesia Gas Laws Anesthesia The kinetic theory of gases makes the following assumptions: Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. It begins by using boyle's law to calculate the volume of oxygen. There are several gas laws that apply to human physiology. By comparison of calculated and measured volumetric. Gas Laws Anesthesia.
From www.studocu.com
05. applied chemistry lecture note Medical Gases Gas Laws The Gas Laws Anesthesia The carefully mixed gas is used to calibrate anesthetic gas monitors. The kinetic theory of gases makes the following assumptions: Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. By comparison of calculated and measured volumetric results it is shown that at atmospheric. There are several gas laws that apply to human physiology. Universal gas constant (r) the universal gas constant (also. Gas Laws Anesthesia.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Gas Laws Anesthesia The document discusses various gas laws and their applications in anesthesia and respiratory physiology. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. By comparison of calculated and measured volumetric results. Gas Laws Anesthesia.
From www.wisegeek.com
What is Anesthetic Gas? (with pictures) Gas Laws Anesthesia The document discusses various gas laws and their applications in anesthesia and respiratory physiology. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. The carefully mixed gas is used to calibrate anesthetic gas monitors. By comparison of calculated and measured volumetric results it is shown that at atmospheric. The kinetic theory of gases makes the following assumptions: Accurate measurements and understanding relationships. Gas Laws Anesthesia.
From www.slideshare.net
gas laws in anesthesia Gas Laws Anesthesia By comparison of calculated and measured volumetric results it is shown that at atmospheric. It begins by using boyle's law to calculate the volume of oxygen. There are several gas laws that apply to human physiology. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. Universal gas constant (r) the universal gas constant (also known. Gas Laws Anesthesia.
From studylib.net
GUIDE NOTES ON GAS LAWS Gas Laws Anesthesia There are several gas laws that apply to human physiology. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. It begins by using boyle's law to calculate the volume of oxygen. By comparison of calculated and measured volumetric results it. Gas Laws Anesthesia.
From www.slideshare.net
gas laws in anesthesia Gas Laws Anesthesia The kinetic theory of gases makes the following assumptions: Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. There are several gas laws that apply to human physiology. By comparison of calculated and measured volumetric results it is shown that at atmospheric. It begins by using boyle's law to calculate the volume of. Gas Laws Anesthesia.
From medicalhubnews.com
Anesthesia And Gas Understanding The Relationship For Safer Medical Gas Laws Anesthesia The document discusses various gas laws and their applications in anesthesia and respiratory physiology. The carefully mixed gas is used to calibrate anesthetic gas monitors. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. It begins by using boyle's law to calculate the volume of oxygen. The kinetic theory of gases makes the following assumptions: By comparison of calculated and measured volumetric. Gas Laws Anesthesia.
From studylib.net
The Ideal and Combined Gas Laws Gas Laws Anesthesia Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. There are several gas laws that apply to human physiology. The carefully mixed gas is used to calibrate anesthetic gas monitors. The kinetic theory of gases makes the following assumptions:. Gas Laws Anesthesia.
From dokumen.tips
(PPT) S.I. units Gases and Gas Laws By Ahmed Ibrahim ; M.D. Prof.of Gas Laws Anesthesia Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. The kinetic theory of gases makes the following assumptions: It begins by using boyle's law to calculate the volume of oxygen. The carefully mixed gas is used to calibrate anesthetic gas monitors. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. By comparison of calculated and measured volumetric. Gas Laws Anesthesia.
From studylib.net
GAS law help sheet Gas Laws Anesthesia Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. By comparison of calculated and measured volumetric results it is shown that at atmospheric. There. Gas Laws Anesthesia.
From www.youtube.com
Gas law triangle explained YouTube Gas Laws Anesthesia The kinetic theory of gases makes the following assumptions: The carefully mixed gas is used to calibrate anesthetic gas monitors. It begins by using boyle's law to calculate the volume of oxygen. By comparison of calculated and measured volumetric results it is shown that at atmospheric. The document discusses various gas laws and their applications in anesthesia and respiratory physiology.. Gas Laws Anesthesia.
From www.slideshare.net
gas laws in anesthesia Gas Laws Anesthesia Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. The document discusses various gas laws and their applications in anesthesia and respiratory physiology. There are several gas laws that apply to human physiology. The kinetic theory of gases makes the following assumptions: By comparison of calculated and measured volumetric results it is shown. Gas Laws Anesthesia.
From www.slideshare.net
gas laws in anesthesia Gas Laws Anesthesia The kinetic theory of gases makes the following assumptions: The document discusses various gas laws and their applications in anesthesia and respiratory physiology. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is. Gas Laws Anesthesia.
From www.scribd.com
Ideal Gas Law 2 PDF Gas Laws Anesthesia Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. By comparison of calculated and measured volumetric results it is shown that at atmospheric. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. It. Gas Laws Anesthesia.
From www.slideshare.net
gas laws in anesthesia Gas Laws Anesthesia Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. The carefully mixed gas is used to calibrate anesthetic gas monitors. The kinetic theory of gases makes the following assumptions: By comparison of calculated and measured volumetric results it is shown that at atmospheric. There are several gas laws that apply to human physiology.. Gas Laws Anesthesia.
From app.pandai.org
Gas Laws Gas Laws Anesthesia The document discusses various gas laws and their applications in anesthesia and respiratory physiology. Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. It begins by using boyle's law to calculate the volume of oxygen. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. Accurate measurements and understanding relationships. Gas Laws Anesthesia.
From www.youtube.com
Gas Laws and respiration YouTube Gas Laws Anesthesia By comparison of calculated and measured volumetric results it is shown that at atmospheric. There are several gas laws that apply to human physiology. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. The document discusses various gas laws and their applications in anesthesia and respiratory physiology.. Gas Laws Anesthesia.
From www.slideshare.net
gas laws in anesthesia Gas Laws Anesthesia Anesthetic gases (nitrous oxide, halothane, isoflurane, desflurane,. Accurate measurements and understanding relationships like between pressure, volume and temperature are important for safe anaesthesia. Universal gas constant (r) the universal gas constant (also known as the molar gas constant and the ideal gas constant) is the. The kinetic theory of gases makes the following assumptions: By comparison of calculated and measured. Gas Laws Anesthesia.