Medical Monitor Definition . Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process.
from www.gehealthcare.com
A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process.
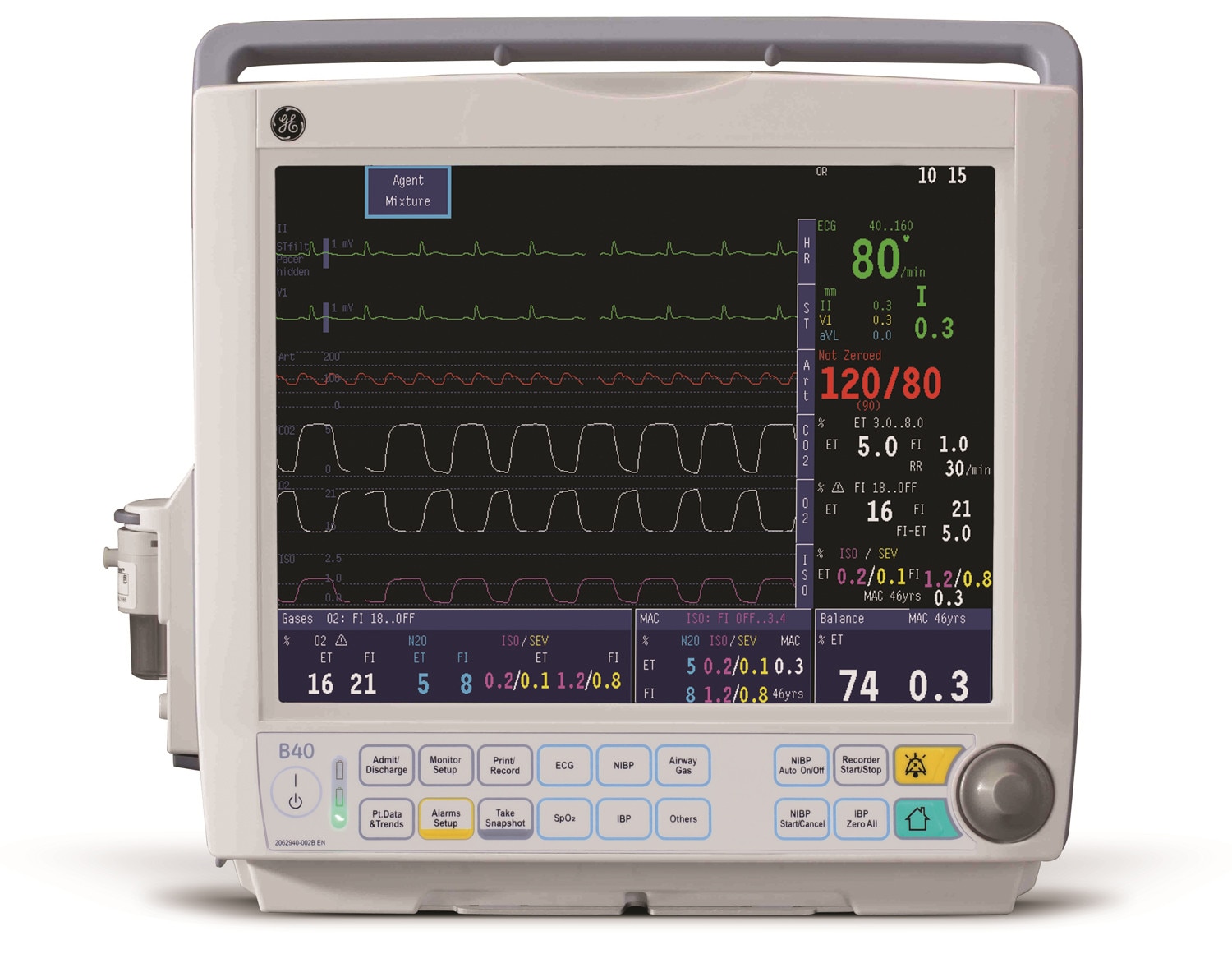
B40 Patient Monitor GE Healthcare
Medical Monitor Definition A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development.
From lifeplusmedical.com
Patient Monitor LifePlus Medical Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe. Medical Monitor Definition.
From www.gehealthcare.com
Patient Monitoring GE Healthcare Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. The e6 good clinical practice (gcp) guidelines define a medical. Medical Monitor Definition.
From blog.agdisplays.com
Medical Monitors An In Depth Look AG Displays Blog Medical Monitor Definition Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products. Medical Monitor Definition.
From www.dreamstime.com
Medical Monitor in the Intensive Care Unit Stock Image Image of Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. A medical monitor acts as the point of reference for study team members. Medical Monitor Definition.
From med.equipment
What types of Patient Monitors are there? MED.equipment Blog Medical Monitor Definition Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Pdf. Medical Monitor Definition.
From www.storyblocks.com
Cinemagraph Of Medical Monitor Inside Stock Footage SBV338410932 Medical Monitor Definition Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. Doctors and medical monitors work together with sponsors and. Medical Monitor Definition.
From blog.atltechnology.com
What are the components of a patient monitoring system? Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. A medical monitor acts as the point of reference for study team members and investigative sites. Medical Monitor Definition.
From www.contecmedsystems.com
Portable High Resolution Medical Sergical 12.1 Inch TFT Color Display Medical Monitor Definition The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. Medical monitors are a critical component of the clinical trial process, providing. Medical Monitor Definition.
From www.medke.com
Definition and classification of Patient monitors Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. A medical monitor acts as the point of reference for study team members. Medical Monitor Definition.
From medmaster.com.ph
M8500 MultiParameter Patient Monitor MEDMASTER Medical Monitor Definition A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. The e6 good clinical practice (gcp) guidelines define a medical monitor as a person. Medical Monitor Definition.
From www.indiamart.com
Medical Monitor, Medical Monitor, Patient Monitor, ICU Monitor Medical Monitor Definition Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and. Medical Monitor Definition.
From www.cardiacdirect.com
How to Read a Patient Monitor Numbers and Lines Explained Medical Monitor Definition Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Pdf | the role of a medical monitor in clinical trials | find, read and cite all. Medical Monitor Definition.
From www.ems1.com
Medical monitoring Guiding EMS differential diagnosis Medical Monitor Definition Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Pdf | the role of a medical monitor in clinical trials | find, read and cite all. Medical Monitor Definition.
From www.dreamstime.com
Heart Rate Monitor in a Hospital Theater. Medical Device for Monitoring Medical Monitor Definition A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Doctors and medical monitors work together with sponsors and clinical. Medical Monitor Definition.
From www.lihnnhs.info
Cardiac Monitor ICU Knowledge Medical Monitor Definition Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products. Medical Monitor Definition.
From www.alamy.com
A medical monitor Stock Photo Alamy Medical Monitor Definition Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. Medical monitors are a critical component of the clinical trial process, providing valuable insights. Medical Monitor Definition.
From www.medistorebd.com
LEE Medical 12.1 Inch MultiParameter Patient Monitor Medical Monitor Definition Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Pdf | the role of a medical monitor in clinical trials | find, read and cite all. Medical Monitor Definition.
From www.dreamstime.com
Medical Monitor in the Operating Room. Stock Image Image of medicine Medical Monitor Definition Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare. Medical Monitor Definition.
From www.cardiacdirect.com
How to Read a Patient Monitor Numbers and Lines Explained Medical Monitor Definition Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. A medical monitor acts as the point of reference for study team members and investigative sites and. Medical Monitor Definition.
From en.njpuao.com
PDJ 3000 Patient Monitor Buy Patient Monitor, Surgical Patient Medical Monitor Definition A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing. Medical Monitor Definition.
From www.dreamstime.com
Regulation of Medical Monitor. Hand of Doctor Stock Photo Image of Medical Monitor Definition Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. The e6 good clinical practice (gcp) guidelines define a medical monitor as a person. Medical Monitor Definition.
From goforward.com
Which Heart Health Monitor Should I Use? Forward Medical Monitor Definition A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Pdf | the role of a medical monitor in clinical trials | find, read. Medical Monitor Definition.
From www.anesplus.com
Choosing the Best Patient Monitor for Your Medical Practice Medical Monitor Definition Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. A medical monitor acts as the point of reference for study team members and investigative. Medical Monitor Definition.
From synergymedco.com
Sony LMD2451MT(LMD2451MT) 24 inch Full HD 3D LCD Medical Monitor Medical Monitor Definition Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the. Medical Monitor Definition.
From tru-vumonitors.com
27" Medical Touch Screen TRUVu Monitors Medical Displays Medical Monitor Definition Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Pdf | the role of a medical monitor in clinical trials | find, read and cite all. Medical Monitor Definition.
From www.gehealthcare.com
B40 Patient Monitor GE Healthcare Medical Monitor Definition Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective. Medical Monitor Definition.
From tru-vumonitors.com
24" Medical Touch Screen Monitor MMZBTP24 Series TRUVu Monitors Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers. Medical Monitor Definition.
From www.dreamstime.com
Modern Medical Monitor with ECG Stock Image Image of data, medicine Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. Doctors and medical monitors work together with sponsors and clinical. Medical Monitor Definition.
From jushadisplay.en.made-in-china.com
1MP 1280X1024 LCD Medical Grade Monitor for X Ray Medical Equipment CE Medical Monitor Definition A medical monitor acts as the point of reference for study team members and investigative sites and determines how to evaluate safety events within a clinical trial. The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. Medical monitors are a critical component of the clinical trial process, providing. Medical Monitor Definition.
From klakxxzqx.blob.core.windows.net
Monitoring Definition In Medical Field at Brian Thurman blog Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers. Medical Monitor Definition.
From amismedical.com
Medical MultiParameter Patient Monitor AMIS MEDICAL Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and. A medical monitor acts as the point of reference for study team members and investigative sites and determines. Medical Monitor Definition.
From en.lepumedical.com
Lepu Medical PC3000 Multi Parameter Patient Monitor Manufacturer Medical Monitor Definition Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Pdf | the role of a medical monitor in clinical trials | find, read and cite all. Medical Monitor Definition.
From www.neweggbusiness.com
What Does Medical Grade Mean for a Computer Monitor? Smart Buyer Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. Generally speaking, safety oversight is the science of collecting, monitoring, researching, assessing and evaluating information from. Medical Monitor Definition.
From www.dreamstime.com
Medical Technology Monitors Stock Photo Image of shock, oxygen 63924240 Medical Monitor Definition The e6 good clinical practice (gcp) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. Medical monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development. Pdf | the role of a medical monitor in clinical trials | find, read and. Medical Monitor Definition.
From www.dreamstime.com
Medical monitor stock photo. Image of digital, hospital 16647622 Medical Monitor Definition Pdf | the role of a medical monitor in clinical trials | find, read and cite all the research you need on researchgate Doctors and medical monitors work together with sponsors and clinical operations teams to develop a safe and effective drug. A medical monitor acts as the point of reference for study team members and investigative sites and determines. Medical Monitor Definition.