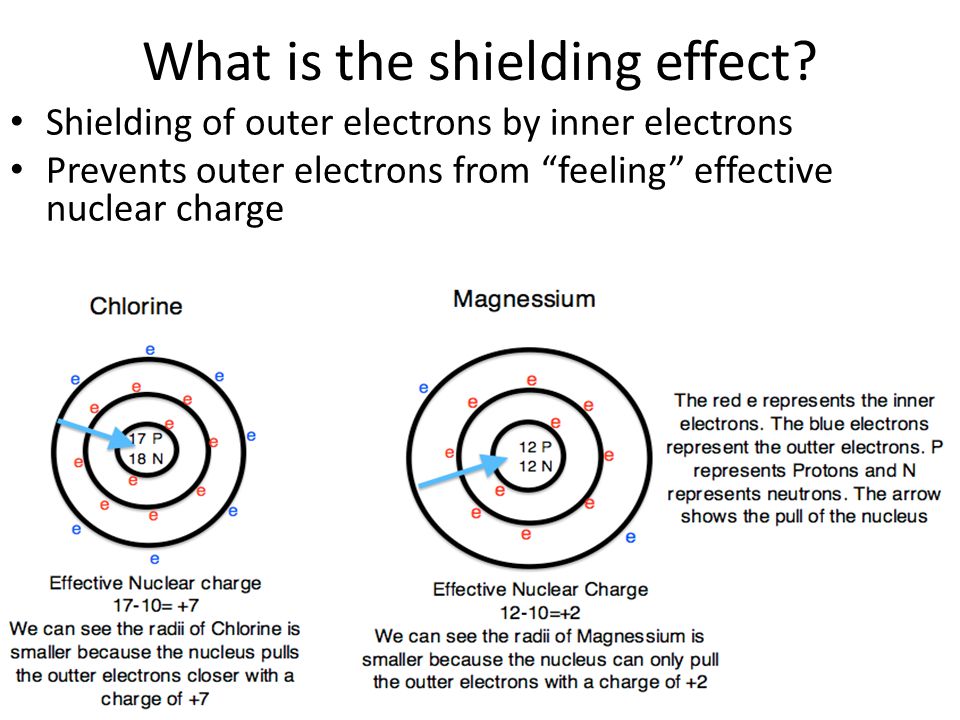
What Is Meant By Shielding Effect . Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer.
from socratic.org
Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital.
How are shielding effect and atomic radius related? Socratic
What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer.
From byjus.com
WHAT IS SHIELDING EFFECT?WHAT IS THE VALUE OF SHIELDING CONSTANT AND What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Shielding refers to the core electrons repelling the outer electrons, which. What Is Meant By Shielding Effect.
From byjus.com
how to calculate the shielding effect What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction. What Is Meant By Shielding Effect.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is Meant By Shielding Effect The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level. What Is Meant By Shielding Effect.
From byjus.com
What is effective nuclear charge and shielding effect? What Is Meant By Shielding Effect The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of. What Is Meant By Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because. What Is Meant By Shielding Effect.
From byjus.com
WHAT IS SHIELDING EFFECT?WHAT IS THE VALUE OF SHIELDING CONSTANT AND What Is Meant By Shielding Effect The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Shielding refers to the core electrons repelling the outer electrons, which lowers the. What Is Meant By Shielding Effect.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy. What Is Meant By Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect refers to the phenomenon where inner shell electrons. What Is Meant By Shielding Effect.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy. What Is Meant By Shielding Effect.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Is Meant By Shielding Effect The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of. What Is Meant By Shielding Effect.
From www.youtube.com
Shielding Effect in the Periodic Table Chemistry YouTube What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction. What Is Meant By Shielding Effect.
From www.youtube.com
What is Shielding Effect in Simple Words Shielding Effect Definition What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of. What Is Meant By Shielding Effect.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID3652853 What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction. What Is Meant By Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is Meant By Shielding Effect The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect increases when elements move down the group in the periodic. What Is Meant By Shielding Effect.
From scienceinfo.com
Shielding effect What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy. What Is Meant By Shielding Effect.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect increases when elements move down the group in the. What Is Meant By Shielding Effect.
From www.youtube.com
Shielding Deshielding Effects Explained Nucleus Get better grade in What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Shielding refers to the core electrons repelling the outer electrons, which. What Is Meant By Shielding Effect.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect increases when elements move down the group in the. What Is Meant By Shielding Effect.
From mungfali.com
What Is Shielding Effect What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because. What Is Meant By Shielding Effect.
From www.youtube.com
418 Shielding Effect YouTube What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect increases when elements move down the group in the. What Is Meant By Shielding Effect.
From www.youtube.com
What does higher shielding effect mean Which element has the highest What Is Meant By Shielding Effect Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which. What Is Meant By Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Meant By Shielding Effect The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of. What Is Meant By Shielding Effect.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. The shielding effect increases when elements move down the group in the periodic table because of an. What Is Meant By Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Meant By Shielding Effect Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. Shielding refers to the core electrons repelling the outer electrons, which lowers the. What Is Meant By Shielding Effect.
From www.youtube.com
What is a shielding effect ? Its Trends in periodic table Chemistry What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect refers to the phenomenon where inner shell electrons partially. What Is Meant By Shielding Effect.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. The shielding effect increases when elements move down the group in the periodic table because of an. What Is Meant By Shielding Effect.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect refers to the phenomenon where inner shell electrons. What Is Meant By Shielding Effect.
From www.youtube.com
Chapter 3 Shielding Effect and Atomic Size YouTube What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction. What Is Meant By Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because. What Is Meant By Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID118383 What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy. What Is Meant By Shielding Effect.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 What Is Meant By Shielding Effect Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Shielding refers to the core electrons repelling the outer electrons, which. What Is Meant By Shielding Effect.
From www.youtube.com
Shielding Effect YouTube What Is Meant By Shielding Effect The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Shielding refers to the core electrons repelling the outer electrons, which. What Is Meant By Shielding Effect.
From mungfali.com
What Is Shielding Effect What Is Meant By Shielding Effect Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The shielding effect refers to the phenomenon where inner shell electrons partially. What Is Meant By Shielding Effect.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Meant By Shielding Effect The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. The shielding effect increases when elements move down the group in the periodic table because of an increase in the number of inner. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level. What Is Meant By Shielding Effect.
From mungfali.com
What Is Shielding Effect What Is Meant By Shielding Effect The shielding effect refers to the phenomenon where inner shell electrons partially block the attraction between the nucleus and the outer. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. The shielding effect increases when elements move down the group in the periodic table because of an. What Is Meant By Shielding Effect.