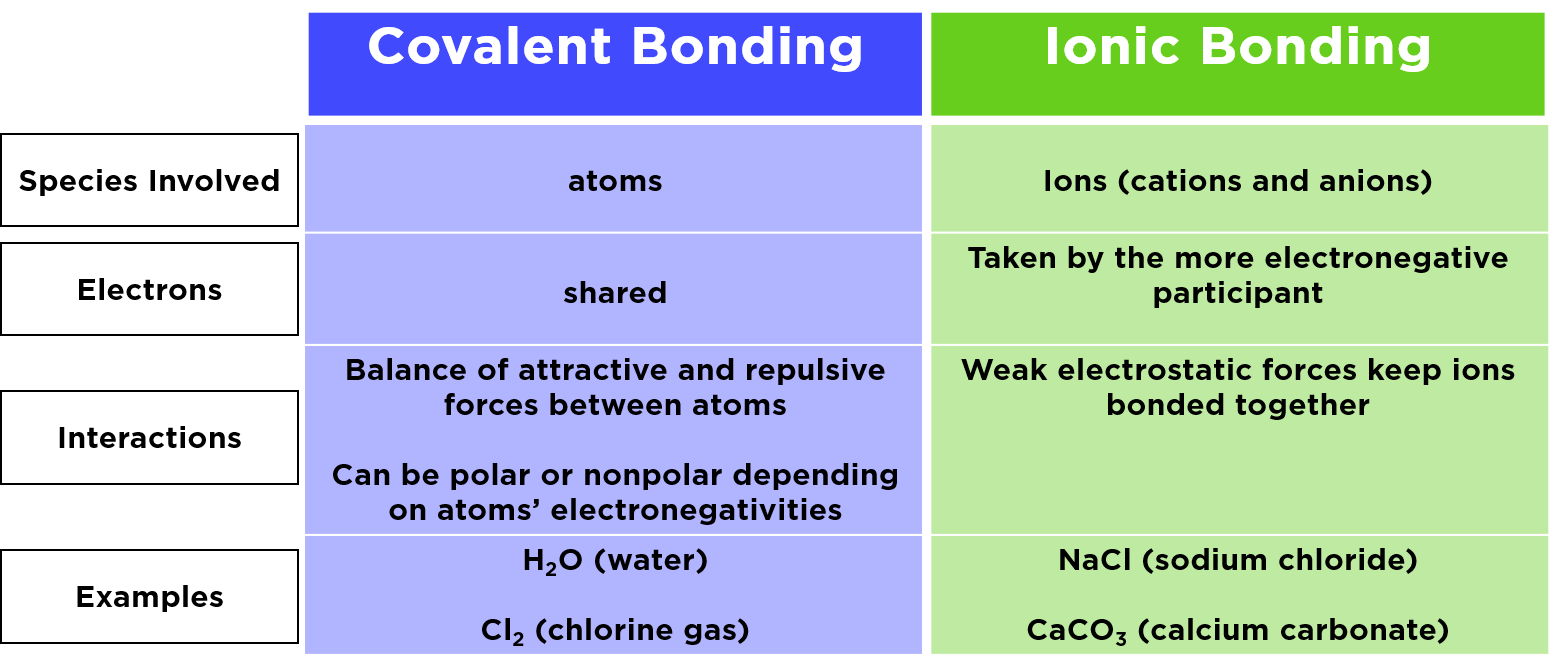
No Is An Example Of Ionic Or Covalent . ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms. Ionic compounds are often solids, and form crystals. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. in ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one. You can often recognize ionic compounds. common table salt is an an example of common compound with ionic bonds.
from www.expii.com
the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. Compounds that do not contain ions, but instead consist of atoms. common table salt is an an example of common compound with ionic bonds. in ionic bonding, atoms transfer electrons to each other. Ionic compounds are often solids, and form crystals. You can often recognize ionic compounds. ionic compounds generally form from metals and nonmetals. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). Ionic bonds require at least one electron donor and one. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic.
Ionic Bond — Formation & Compounds Expii
No Is An Example Of Ionic Or Covalent common table salt is an an example of common compound with ionic bonds. common table salt is an an example of common compound with ionic bonds. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). Ionic compounds are often solids, and form crystals. Ionic bonds require at least one electron donor and one. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. You can often recognize ionic compounds. ionic compounds generally form from metals and nonmetals. in ionic bonding, atoms transfer electrons to each other. Compounds that do not contain ions, but instead consist of atoms.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart No Is An Example Of Ionic Or Covalent the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. Compounds that do not contain ions, but instead consist of atoms. in ionic bonding, atoms transfer electrons to each other. however, it is not always true (for example, aluminum chloride, alcl 3, is not. No Is An Example Of Ionic Or Covalent.
From www.pinterest.com
An example of covalent bonding between hydrogen and chlorine. Instead No Is An Example Of Ionic Or Covalent Ionic compounds are often solids, and form crystals. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. in ionic bonding, atoms transfer. No Is An Example Of Ionic Or Covalent.
From examples.yourdictionary.com
Main Differences Between Ionic and Covalent Bonds YourDictionary No Is An Example Of Ionic Or Covalent the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). ionic compounds generally form from metals and nonmetals. the key difference between an ionic and covalent bond is that one. No Is An Example Of Ionic Or Covalent.
From pediaa.com
Difference Between Covalent and Ionic Bonds No Is An Example Of Ionic Or Covalent the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. common table salt is an an example of common compound with ionic bonds. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). Ionic compounds are often solids, and form crystals.. No Is An Example Of Ionic Or Covalent.
From quizlet.com
Ionic and Covalent Bonds Diagram Quizlet No Is An Example Of Ionic Or Covalent ionic compounds generally form from metals and nonmetals. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. Ionic compounds are often solids, and form crystals. Ionic bonds require. No Is An Example Of Ionic Or Covalent.
From blogs.glowscotland.org.uk
Review Comparing Ionic/ Covalent Compounds S3 Chemistry Consolidation No Is An Example Of Ionic Or Covalent the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. in ionic bonding, atoms transfer electrons to each other. Ionic bonds require at. No Is An Example Of Ionic Or Covalent.
From www.expii.com
Ionic Bond — Formation & Compounds Expii No Is An Example Of Ionic Or Covalent You can often recognize ionic compounds. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). common table salt is an an example of common compound with ionic bonds. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. Ionic compounds. No Is An Example Of Ionic Or Covalent.
From www.coursehero.com
[Solved] I. Identifying if Ionic or Covalent bond and show how they No Is An Example Of Ionic Or Covalent common table salt is an an example of common compound with ionic bonds. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). Ionic bonds require at least one electron donor and one. Ionic compounds are often solids, and form crystals. ionic compounds generally form from metals and nonmetals. the degree. No Is An Example Of Ionic Or Covalent.
From stock.adobe.com
Vecteur Stock Ionic covalent bonds examples. Chemical structural models No Is An Example Of Ionic Or Covalent Ionic bonds require at least one electron donor and one. common table salt is an an example of common compound with ionic bonds. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. the degree to which electrons are shared between atoms varies from. No Is An Example Of Ionic Or Covalent.
From www.myxxgirl.com
Difference Between Ionic Covalent And Metallic Bonds Definition My No Is An Example Of Ionic Or Covalent Ionic bonds require at least one electron donor and one. in ionic bonding, atoms transfer electrons to each other. common table salt is an an example of common compound with ionic bonds. Compounds that do not contain ions, but instead consist of atoms. ionic compounds generally form from metals and nonmetals. the key difference between an. No Is An Example Of Ionic Or Covalent.
From clarence-well-duffy.blogspot.com
Explain the Major Differences Between Covalent and Ionic Bonding No Is An Example Of Ionic Or Covalent however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). Ionic bonds require at least one electron donor and one. Compounds that do not contain ions, but instead consist of atoms. You can often recognize ionic compounds. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding). No Is An Example Of Ionic Or Covalent.
From www.youtube.com
Ionic and Covalent Bonding Chemistry YouTube No Is An Example Of Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). Ionic bonds require at least one electron donor and one. . No Is An Example Of Ionic Or Covalent.
From slideplayer.com
Crystal Binding (Bonding) Part III ppt download No Is An Example Of Ionic Or Covalent ionic compounds generally form from metals and nonmetals. You can often recognize ionic compounds. in ionic bonding, atoms transfer electrons to each other. Compounds that do not contain ions, but instead consist of atoms. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). the degree to which electrons are shared. No Is An Example Of Ionic Or Covalent.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary No Is An Example Of Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. ionic compounds. No Is An Example Of Ionic Or Covalent.
From www.shutterstock.com
5 Ionic Vs Covalent Compounds Images, Stock Photos & Vectors Shutterstock No Is An Example Of Ionic Or Covalent ionic compounds generally form from metals and nonmetals. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. Compounds that do not contain ions, but instead consist of atoms. the key difference between an ionic and covalent bond is that one atom essentially donates an electron. No Is An Example Of Ionic Or Covalent.
From byjus.com
Is CCl4 Covalent or Ionic? No Is An Example Of Ionic Or Covalent You can often recognize ionic compounds. in ionic bonding, atoms transfer electrons to each other. Ionic compounds are often solids, and form crystals. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. Ionic bonds require at least one electron donor and one. the. No Is An Example Of Ionic Or Covalent.
From sciencenotes.org
Ionic vs Covalent Bonds No Is An Example Of Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms. common table salt is an an example of common compound with ionic bonds. in ionic bonding, atoms transfer electrons to each other. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. the key. No Is An Example Of Ionic Or Covalent.
From slideplayer.com
Ionic & Covalent Review ppt download No Is An Example Of Ionic Or Covalent common table salt is an an example of common compound with ionic bonds. Compounds that do not contain ions, but instead consist of atoms. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. however, it is not always true (for example, aluminum chloride,. No Is An Example Of Ionic Or Covalent.
From psiberg.com
Ionic vs. Covalent Bond A Definitive Comparison PSIBERG No Is An Example Of Ionic Or Covalent You can often recognize ionic compounds. Ionic compounds are often solids, and form crystals. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). common table salt is an. No Is An Example Of Ionic Or Covalent.
From slideplayer.com
The Nature of Matter. ppt download No Is An Example Of Ionic Or Covalent however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in. No Is An Example Of Ionic Or Covalent.
From slideplayer.com
Overview Bonding, Structure and the properties of matter ppt download No Is An Example Of Ionic Or Covalent Ionic bonds require at least one electron donor and one. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. ionic compounds generally form from metals and nonmetals. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to. No Is An Example Of Ionic Or Covalent.
From studylib.net
Ionic and Covalent Worksheet No Is An Example Of Ionic Or Covalent ionic compounds generally form from metals and nonmetals. common table salt is an an example of common compound with ionic bonds. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. Ionic compounds are often solids, and form crystals. the degree to which. No Is An Example Of Ionic Or Covalent.
From www.jagranjosh.com
What Is The Difference Between Ionic And Covalent Bonds? No Is An Example Of Ionic Or Covalent common table salt is an an example of common compound with ionic bonds. in ionic bonding, atoms transfer electrons to each other. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). Compounds that do not contain ions, but instead consist of atoms. ionic compounds generally form from metals and nonmetals.. No Is An Example Of Ionic Or Covalent.
From giovannigokesalas.blogspot.com
Difference Between Ionic and Covalent Bonds No Is An Example Of Ionic Or Covalent the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. in ionic bonding, atoms transfer electrons to each other. Ionic bonds require at. No Is An Example Of Ionic Or Covalent.
From kidsworksheetfun.com
Identifying Ionic And Covalent Bonds Worksheet Answers Kidsworksheetfun No Is An Example Of Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. in ionic bonding, atoms transfer electrons to each other. ionic compounds generally form from metals and nonmetals. however, it is not always. No Is An Example Of Ionic Or Covalent.
From www.teachoo.com
[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo No Is An Example Of Ionic Or Covalent the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. in ionic bonding, atoms transfer electrons to each other. Ionic compounds are often solids, and form crystals. Compounds that do not contain ions, but instead consist of atoms. ionic compounds generally form from metals and nonmetals.. No Is An Example Of Ionic Or Covalent.
From www.differencebetween.com
Difference Between Ionic and Covalent Compounds Compare the No Is An Example Of Ionic Or Covalent the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. common table salt is an an example of common compound with ionic bonds. Compounds that do not contain ions, but instead consist of atoms. You can often recognize ionic compounds. Ionic compounds are often solids,. No Is An Example Of Ionic Or Covalent.
From slideplayer.com
Biochemical Aspect of Biology ppt download No Is An Example Of Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. common table salt is an an example of common compound with ionic bonds. Ionic bonds require at least one electron donor and one. in ionic. No Is An Example Of Ionic Or Covalent.
From ar.inspiredpencil.com
Covalent Bonding Vs Ionic Bonding No Is An Example Of Ionic Or Covalent Ionic bonds require at least one electron donor and one. Compounds that do not contain ions, but instead consist of atoms. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. Ionic compounds are often solids, and form crystals. however, it is not always true (for example,. No Is An Example Of Ionic Or Covalent.
From fourthgradegc.blogspot.com
Fourth Grade GC August 2013 No Is An Example Of Ionic Or Covalent in ionic bonding, atoms transfer electrons to each other. Compounds that do not contain ions, but instead consist of atoms. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. ionic compounds generally form from metals and nonmetals. Ionic bonds require at least one. No Is An Example Of Ionic Or Covalent.
From sciencenotes.org
Naming Covalent Compounds Nomenclature Rules No Is An Example Of Ionic Or Covalent common table salt is an an example of common compound with ionic bonds. You can often recognize ionic compounds. Compounds that do not contain ions, but instead consist of atoms. however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). in ionic bonding, atoms transfer electrons to each other. ionic compounds. No Is An Example Of Ionic Or Covalent.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii No Is An Example Of Ionic Or Covalent Ionic bonds require at least one electron donor and one. in ionic bonding, atoms transfer electrons to each other. ionic compounds generally form from metals and nonmetals. common table salt is an an example of common compound with ionic bonds. Ionic compounds are often solids, and form crystals. the degree to which electrons are shared between. No Is An Example Of Ionic Or Covalent.
From worksheetlibbader.z19.web.core.windows.net
Worksheet Chemical Bonding Ionic & Covalent No Is An Example Of Ionic Or Covalent however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. Ionic compounds are often solids, and form crystals. ionic compounds generally form from metals and nonmetals. in ionic. No Is An Example Of Ionic Or Covalent.
From slideplayer.com
Chapter 6 Chemical Reactions Occur in Predictable Ways ppt download No Is An Example Of Ionic Or Covalent however, it is not always true (for example, aluminum chloride, alcl 3, is not ionic). Compounds that do not contain ions, but instead consist of atoms. the degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic. You can often recognize ionic compounds. ionic compounds generally form. No Is An Example Of Ionic Or Covalent.
From sciencenotes.org
Compounds With Both Ionic and Covalent Bonds No Is An Example Of Ionic Or Covalent the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. You can often recognize ionic compounds. Ionic bonds require at least one electron donor and one. common table salt is an an example of common compound with ionic bonds. Compounds that do not contain ions,. No Is An Example Of Ionic Or Covalent.