What Happens When You Add A Strong Base To A Weak Acid . Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: The next diagram shows the ph curve for adding a strong acid to a strong base. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). A solution of acetic acid (\ (\ce. In a typical titration, a few drops of indicator, such as. As strong base is added, an icf chart. The reaction of the weak acid, acetic acid, with a strong base, naoh,. Initially, an ice chart is used to find the ph of the weak acid. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Strong acid v strong base.
from www.expii.com
A solution of acetic acid (\ (\ce. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: Initially, an ice chart is used to find the ph of the weak acid. Strong acid v strong base. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. As strong base is added, an icf chart. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). The reaction of the weak acid, acetic acid, with a strong base, naoh,.
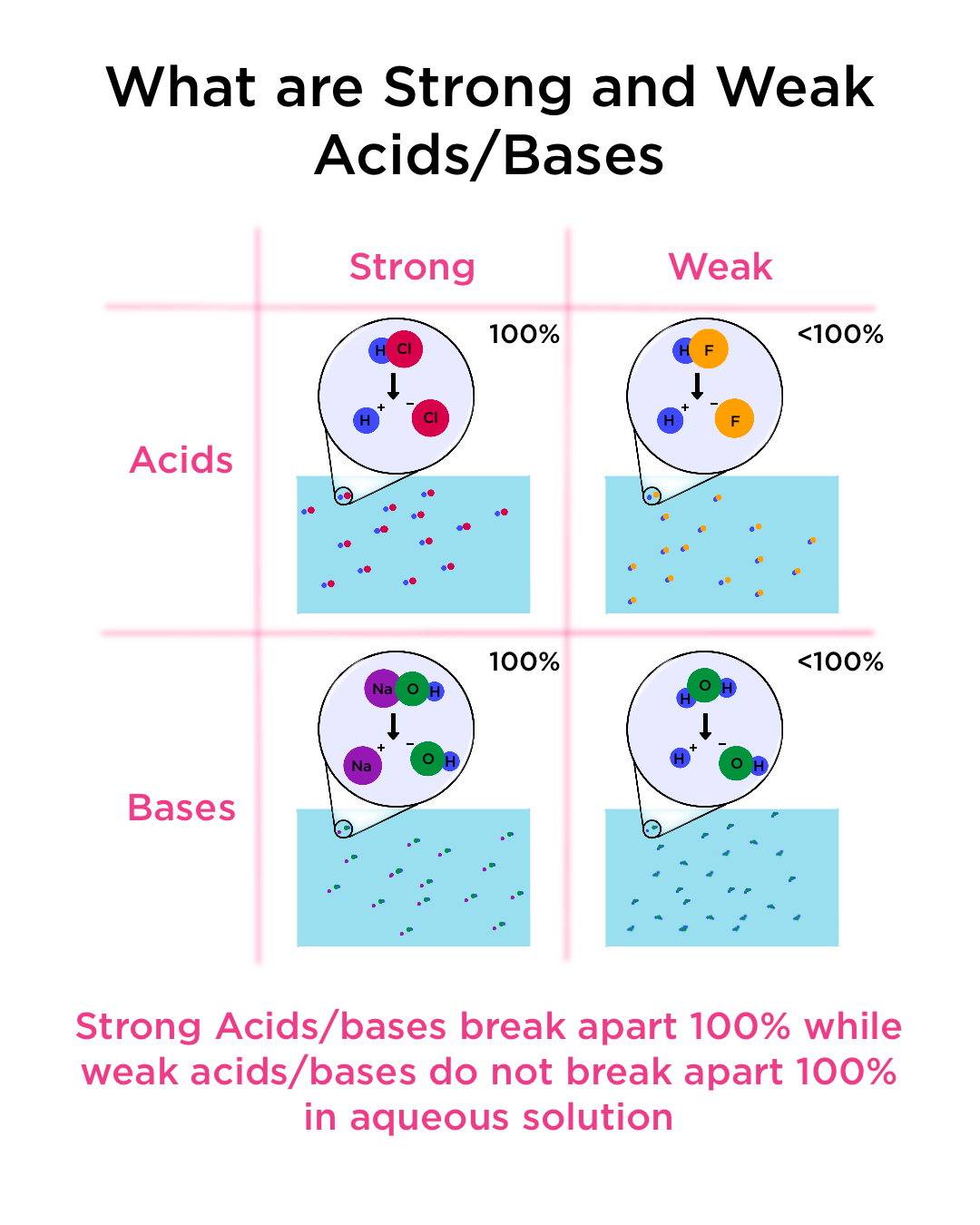
Strong and Weak Acids and Bases — Definition & Examples Expii
What Happens When You Add A Strong Base To A Weak Acid Strong acid v strong base. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). In a typical titration, a few drops of indicator, such as. The next diagram shows the ph curve for adding a strong acid to a strong base. Initially, an ice chart is used to find the ph of the weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A solution of acetic acid (\ (\ce. As strong base is added, an icf chart. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: Strong acid v strong base. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). The reaction of the weak acid, acetic acid, with a strong base, naoh,.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry What Happens When You Add A Strong Base To A Weak Acid Superimposed on it are the ph ranges for methyl orange and phenolphthalein. As strong base is added, an icf chart. Strong acid v strong base. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: In a typical titration, a few drops. What Happens When You Add A Strong Base To A Weak Acid.
From www.pinterest.com
Strong and Weak Acids & Bases Chemistry classroom, Ap chemistry What Happens When You Add A Strong Base To A Weak Acid Strong acid v strong base. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). In a typical titration, a few drops of indicator, such as. As strong base is added, an icf chart. The next diagram shows the ph curve for adding a strong acid to a strong base.. What Happens When You Add A Strong Base To A Weak Acid.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo What Happens When You Add A Strong Base To A Weak Acid A solution of acetic acid (\ (\ce. The next diagram shows the ph curve for adding a strong acid to a strong base. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the. What Happens When You Add A Strong Base To A Weak Acid.
From www.teachoo.com
Difference between Strong and Weak Base with Examples [in Table] What Happens When You Add A Strong Base To A Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: In a typical titration, a few drops of indicator, such as. Superimposed on it are the. What Happens When You Add A Strong Base To A Weak Acid.
From www.numerade.com
SOLVEDHow is an acidic salt formed? 0 A the reaction of a strong acid What Happens When You Add A Strong Base To A Weak Acid In a typical titration, a few drops of indicator, such as. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Superimposed. What Happens When You Add A Strong Base To A Weak Acid.
From pediaa.com
Difference Between Strong and Weak Acids Definition, Properties, Examples What Happens When You Add A Strong Base To A Weak Acid Initially, an ice chart is used to find the ph of the weak acid. Strong acid v strong base. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: The titration of a weak acid with a strong base involves the direct. What Happens When You Add A Strong Base To A Weak Acid.
From www.teachoo.com
Why do HCl, HNO3, etc., show acidic characters in aqueous solutions What Happens When You Add A Strong Base To A Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. In a typical titration, a few drops of indicator, such as. Initially, an ice chart is used to find the ph of the weak acid. If a strong acid is added to a buffer, the weak base will react with the h + from. What Happens When You Add A Strong Base To A Weak Acid.
From www.expii.com
The pH of Weak Acid and Base Solutions — Examples Expii What Happens When You Add A Strong Base To A Weak Acid In a typical titration, a few drops of indicator, such as. As strong base is added, an icf chart. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. If a strong acid is. What Happens When You Add A Strong Base To A Weak Acid.
From www.youtube.com
Weak acidstrong base reactions Acids and bases AP Chemistry Khan What Happens When You Add A Strong Base To A Weak Acid As strong base is added, an icf chart. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Initially, an ice chart is used to find the ph of the weak acid. The reaction of the weak acid, acetic acid, with a strong base, naoh,. The next diagram shows the ph curve for adding a strong acid to. What Happens When You Add A Strong Base To A Weak Acid.
From www.goodscience.com.au
Acids, Bases and pH Good Science What Happens When You Add A Strong Base To A Weak Acid In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). Initially, an ice chart is used to find the ph of the weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Buffer solutions. What Happens When You Add A Strong Base To A Weak Acid.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 What Happens When You Add A Strong Base To A Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. Initially, an ice chart is used to find the ph of the weak acid. The reaction of the weak acid, acetic acid, with a strong base, naoh,. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Buffer solutions resist a change. What Happens When You Add A Strong Base To A Weak Acid.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation What Happens When You Add A Strong Base To A Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. A solution of acetic acid (\ (\ce. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). The. What Happens When You Add A Strong Base To A Weak Acid.
From www.slideserve.com
PPT Ch.9 J.C. Rowe PowerPoint Presentation, free download ID3493976 What Happens When You Add A Strong Base To A Weak Acid In a typical titration, a few drops of indicator, such as. The next diagram shows the ph curve for adding a strong acid to a strong base. As strong base is added, an icf chart. A solution of acetic acid (\ (\ce. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base. What Happens When You Add A Strong Base To A Weak Acid.
From www.thoughtco.com
List of Common Strong and Weak Acids What Happens When You Add A Strong Base To A Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). The next diagram shows the ph curve for adding a strong acid. What Happens When You Add A Strong Base To A Weak Acid.
From studylib.net
Titration Curve weak base with strong acid START What Happens When You Add A Strong Base To A Weak Acid In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Strong acid v strong base. Buffer solutions resist a change in ph when small amounts of. What Happens When You Add A Strong Base To A Weak Acid.
From www.slideserve.com
PPT Ionization Constants of Acids and Bases Strengths of Acids and What Happens When You Add A Strong Base To A Weak Acid If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: The reaction of the weak acid, acetic acid, with a strong base, naoh,. In a typical titration, a few drops of indicator, such as. The titration of a weak acid with a. What Happens When You Add A Strong Base To A Weak Acid.
From pediaa.com
Difference Between Strong and Weak Bases Definition, Properties What Happens When You Add A Strong Base To A Weak Acid Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). The reaction of the weak acid, acetic acid, with a strong base, naoh,. As strong base is added, an icf chart. In a typical titration, a few drops of indicator, such as. Initially, an ice chart. What Happens When You Add A Strong Base To A Weak Acid.
From www.slideserve.com
PPT Unit 3 PowerPoint Presentation, free download ID2956486 What Happens When You Add A Strong Base To A Weak Acid A solution of acetic acid (\ (\ce. As strong base is added, an icf chart. In a typical titration, a few drops of indicator, such as. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). The reaction of the weak acid, acetic acid, with a. What Happens When You Add A Strong Base To A Weak Acid.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps What Happens When You Add A Strong Base To A Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh,. In a typical titration, a few drops of indicator, such as. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). A solution of acetic acid (\ (\ce. The titration of a weak acid with a strong. What Happens When You Add A Strong Base To A Weak Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts What Happens When You Add A Strong Base To A Weak Acid In a typical titration, a few drops of indicator, such as. The next diagram shows the ph curve for adding a strong acid to a strong base. A solution of acetic acid (\ (\ce. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). Strong acid. What Happens When You Add A Strong Base To A Weak Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts What Happens When You Add A Strong Base To A Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). A solution of acetic acid (\ (\ce. As strong base is added,. What Happens When You Add A Strong Base To A Weak Acid.
From general.chemistrysteps.com
Acidity of a Salt Solution Chemistry Steps What Happens When You Add A Strong Base To A Weak Acid In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Strong acid v strong. What Happens When You Add A Strong Base To A Weak Acid.
From studylib.net
Strong and weak acids and bases What Happens When You Add A Strong Base To A Weak Acid In a typical titration, a few drops of indicator, such as. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). As strong base is added, an icf chart. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. What Happens When You Add A Strong Base To A Weak Acid.
From shiken.ai
Weak Acids and Bases What Happens When You Add A Strong Base To A Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Strong acid v strong base. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). A solution of. What Happens When You Add A Strong Base To A Weak Acid.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases What Happens When You Add A Strong Base To A Weak Acid A solution of acetic acid (\ (\ce. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Superimposed on it are the ph ranges for methyl. What Happens When You Add A Strong Base To A Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps What Happens When You Add A Strong Base To A Weak Acid As strong base is added, an icf chart. The next diagram shows the ph curve for adding a strong acid to a strong base. In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). Buffer solutions resist a change in ph when small amounts of a strong acid or a. What Happens When You Add A Strong Base To A Weak Acid.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID What Happens When You Add A Strong Base To A Weak Acid Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Initially, an ice chart is used to find the ph of the weak acid. As strong base is added, an icf chart. Strong acid v strong base. The next diagram shows the ph curve for adding a strong acid to a strong base. If a strong acid is. What Happens When You Add A Strong Base To A Weak Acid.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts What Happens When You Add A Strong Base To A Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a strong base, naoh,. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to. What Happens When You Add A Strong Base To A Weak Acid.
From sciencenotes.org
List of Common Strong and Weak Acids What Happens When You Add A Strong Base To A Weak Acid In a typical titration, a few drops of indicator, such as. A solution of acetic acid (\ (\ce. The next diagram shows the ph curve for adding a strong acid to a strong base. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak. What Happens When You Add A Strong Base To A Weak Acid.
From www.expii.com
Strong and Weak Acids and Bases — Definition & Examples Expii What Happens When You Add A Strong Base To A Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh,. A solution of acetic acid (\ (\ce. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. The next diagram shows the ph curve for adding a strong acid to a strong base. As strong base is added, an icf chart. Strong acid v strong. What Happens When You Add A Strong Base To A Weak Acid.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves What Happens When You Add A Strong Base To A Weak Acid In a typical titration, a few drops of indicator, such as. The reaction of the weak acid, acetic acid, with a strong base, naoh,. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Buffer solutions resist a change in ph when small amounts of a. What Happens When You Add A Strong Base To A Weak Acid.
From www.pinterest.ca
Strong and weak acids collection with educational diagram outline What Happens When You Add A Strong Base To A Weak Acid In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). The next diagram shows the ph curve for adding a strong acid to a strong base. The reaction of the weak acid, acetic acid, with a strong base, naoh,. The titration of a weak acid with a strong base involves. What Happens When You Add A Strong Base To A Weak Acid.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 What Happens When You Add A Strong Base To A Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. The reaction of the weak acid, acetic acid, with a strong base, naoh,. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Strong acid v strong base. Initially, an. What Happens When You Add A Strong Base To A Weak Acid.
From www.teachoo.com
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions What Happens When You Add A Strong Base To A Weak Acid In this particular case, the weak base (colored in green), is being titrated by the strong acid (colored in red). A solution of acetic acid (\ (\ce. Strong acid v strong base. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha:. What Happens When You Add A Strong Base To A Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps What Happens When You Add A Strong Base To A Weak Acid A solution of acetic acid (\ (\ce. In a typical titration, a few drops of indicator, such as. As strong base is added, an icf chart. Strong acid v strong base. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: The. What Happens When You Add A Strong Base To A Weak Acid.