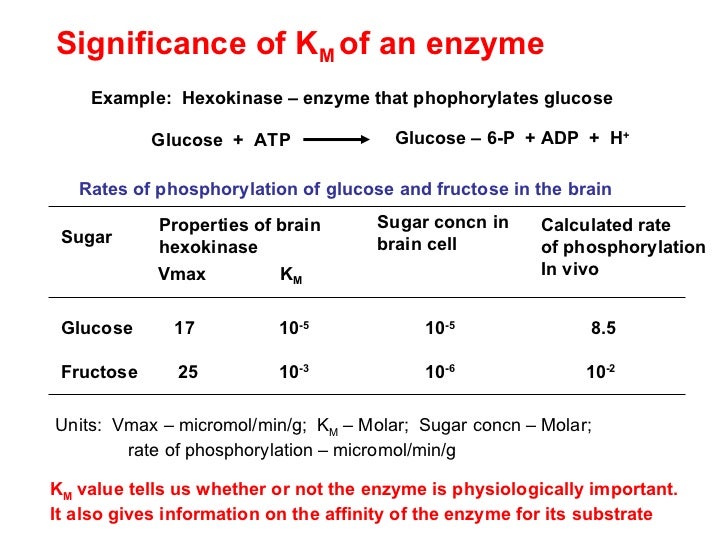
Enzyme Km Binding Affinity . Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. An enzyme's k m describes the substrate. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. Measurement of km depends on the measurement of vmax. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. Enzymes have varying tendencies to bind their substrates (affinities). What it measures, in simple terms, is the affinity an enzyme has for its substrate. Dive into the biochemical reactions that govern enzyme.
from es.slideshare.net
Measurement of km depends on the measurement of vmax. \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. Enzymes have varying tendencies to bind their substrates (affinities). What it measures, in simple terms, is the affinity an enzyme has for its substrate. Dive into the biochemical reactions that govern enzyme. Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. An enzyme's k m describes the substrate. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand.
7.29.10 enzymes coloso
Enzyme Km Binding Affinity Dive into the biochemical reactions that govern enzyme. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. Dive into the biochemical reactions that govern enzyme. An enzyme's k m describes the substrate. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. Measurement of km depends on the measurement of vmax. Enzymes have varying tendencies to bind their substrates (affinities). What it measures, in simple terms, is the affinity an enzyme has for its substrate. \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its.
From slideplayer.com
Enzyme 9/1/ ppt download Enzyme Km Binding Affinity \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. What it measures, in simple terms, is the affinity an enzyme has for its substrate. Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. Dive. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID903733 Enzyme Km Binding Affinity Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. Dive into the biochemical reactions that govern enzyme. \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. The km. Enzyme Km Binding Affinity.
From www.biologyonline.com
Vmax Definition and Examples Biology Online Dictionary Enzyme Km Binding Affinity Enzymes have varying tendencies to bind their substrates (affinities). An enzyme's k m describes the substrate. Measurement of km depends on the measurement of vmax. Dive into the biochemical reactions that govern enzyme. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. Two. Enzyme Km Binding Affinity.
From www.numerade.com
SOLVED BIO 1005 VI. Which of these is an approximate measure of enzymesubstrate binding Enzyme Km Binding Affinity Enzymes have varying tendencies to bind their substrates (affinities). Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. \[ e + s \xrightarrow[ ]{k_1}[ es. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 Enzyme Km Binding Affinity Dive into the biochemical reactions that govern enzyme. Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. Enzymes have varying tendencies to bind their substrates. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Lecture Notes for Chapter 7 Enzyme and Inhibition PowerPoint Presentation ID Enzyme Km Binding Affinity Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km). Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID305372 Enzyme Km Binding Affinity \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. Enzymes have varying tendencies to bind their substrates (affinities). Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. The nmt1 example highlights the crucial role. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 Enzyme Km Binding Affinity An enzyme's k m describes the substrate. What it measures, in simple terms, is the affinity an enzyme has for its substrate. Measurement of km depends on the measurement of vmax. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. The km is a measure of. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Km Measure of binding affinity (roughly) The lower the Km, the tighter the binding Vmax Enzyme Km Binding Affinity Measurement of km depends on the measurement of vmax. Enzymes have varying tendencies to bind their substrates (affinities). An enzyme's k m describes the substrate. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. Affinities of enzymes for substrates vary considerably, so knowing. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID2045841 Enzyme Km Binding Affinity Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. Enzymes have varying tendencies to bind their substrates (affinities). What it measures, in simple terms, is the affinity an enzyme has for its substrate. \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Km Measure of binding affinity (roughly) The lower the Km, the tighter the binding Vmax Enzyme Km Binding Affinity An enzyme's k m describes the substrate. \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. What it measures,. Enzyme Km Binding Affinity.
From slideplayer.com
Lab Activity 6 Enzyme ppt download Enzyme Km Binding Affinity The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. Measurement of km depends on the measurement of vmax. What it measures, in simple terms, is the affinity an enzyme has for its substrate. Enzymes have varying tendencies to bind their substrates (affinities). Affinities. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID903733 Enzyme Km Binding Affinity Enzymes have varying tendencies to bind their substrates (affinities). An enzyme's k m describes the substrate. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and. Enzyme Km Binding Affinity.
From www.researchgate.net
Enzyme activity and binding affinity of an inhibitor (DDC) of hECSOD f... Download Scientific Enzyme Km Binding Affinity What it measures, in simple terms, is the affinity an enzyme has for its substrate. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. An enzyme's k m describes the substrate. Measurement of km depends on the measurement of vmax. Dive into the biochemical reactions that. Enzyme Km Binding Affinity.
From slideplayer.com
Enzymes. ppt download Enzyme Km Binding Affinity What it measures, in simple terms, is the affinity an enzyme has for its substrate. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report.. Enzyme Km Binding Affinity.
From www.reddit.com
Bigger Km means bigger affinity of enzyme for substrate or smaller affinity of enzyme for Enzyme Km Binding Affinity Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. Dive into the biochemical reactions that govern enzyme. \[ e + s \xrightarrow[ ]{k_1}[ es ]. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT LECTURE 2 ENZYME PowerPoint Presentation, free download ID5086606 Enzyme Km Binding Affinity Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. Measurement of km depends on the measurement of vmax. What it measures, in simple terms, is the affinity an enzyme has for its substrate. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or. Enzyme Km Binding Affinity.
From www.researchgate.net
Measurements of binding affinity and enzyme Measurements of... Download Scientific Enzyme Km Binding Affinity What it measures, in simple terms, is the affinity an enzyme has for its substrate. Enzymes have varying tendencies to bind their substrates (affinities). \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. Dive into the biochemical reactions that govern enzyme. Measurement of km. Enzyme Km Binding Affinity.
From www.youtube.com
Lecture 4C EnzymeSubstrate Binding YouTube Enzyme Km Binding Affinity The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. An enzyme's k m describes the substrate. What it measures, in simple terms, is the affinity an enzyme has for its substrate. Affinities of enzymes for substrates vary considerably, so knowing km helps us. Enzyme Km Binding Affinity.
From www.researchgate.net
Fig. E.1. Changes in enzyme affinity upon competition. (a) Lognormal... Download Scientific Enzyme Km Binding Affinity The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. Two commonly encountered parameters in enzyme kinetics are the michaelis constant. Enzyme Km Binding Affinity.
From slidetodoc.com
Regulation of Metabolism Regulation of Metabolism How does Enzyme Km Binding Affinity Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. Measurement of km depends on the measurement of vmax. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. Dive into the biochemical reactions that govern. Enzyme Km Binding Affinity.
From exobdajhi.blob.core.windows.net
Kd Binding Affinity Calculation at Durand blog Enzyme Km Binding Affinity \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. Dive into the biochemical reactions that govern enzyme. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. The. Enzyme Km Binding Affinity.
From www.researchgate.net
Binding affinity and enzyme experiments studied by NMR a... Download Scientific Diagram Enzyme Km Binding Affinity An enzyme's k m describes the substrate. Dive into the biochemical reactions that govern enzyme. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Chapter 6 Enzymes PowerPoint Presentation, free download ID5869201 Enzyme Km Binding Affinity Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. What it measures, in simple terms, is the affinity an enzyme has for its substrate. Dive into the biochemical reactions that govern enzyme. Measurement of km depends on the measurement of vmax. \[ e +. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Enzymes/ PowerPoint Presentation, free download ID1102748 Enzyme Km Binding Affinity Measurement of km depends on the measurement of vmax. An enzyme's k m describes the substrate. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in. Enzyme Km Binding Affinity.
From es.slideshare.net
7.29.10 enzymes coloso Enzyme Km Binding Affinity Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. Enzymes have varying tendencies to bind their substrates (affinities). Measurement of km depends on the measurement. Enzyme Km Binding Affinity.
From www.researchgate.net
Binding affinity versus enzyme activity. The enzyme activity of active... Download Scientific Enzyme Km Binding Affinity Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how. Enzyme Km Binding Affinity.
From teachmephysiology.com
Enzyme Structure Function MichaelisMenten Enzyme Km Binding Affinity \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. An enzyme's k m describes the substrate. Two commonly encountered parameters in. Enzyme Km Binding Affinity.
From courses.lumenlearning.com
Enzymes OpenStax Biology 2e Enzyme Km Binding Affinity An enzyme's k m describes the substrate. Enzymes have varying tendencies to bind their substrates (affinities). The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. Dive into the biochemical reactions that govern enzyme. The km is a measure of the binding affinity of an enzyme for. Enzyme Km Binding Affinity.
From slideplayer.com
Protein Engineering Protein engineering Industrial enzymes (Table 8.1) ppt download Enzyme Km Binding Affinity Measurement of km depends on the measurement of vmax. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to the substrate being used. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. Two commonly encountered. Enzyme Km Binding Affinity.
From www.slideserve.com
PPT Chapter 15 Enzymatic Catalysis PowerPoint Presentation ID484310 Enzyme Km Binding Affinity Enzymes have varying tendencies to bind their substrates (affinities). An enzyme's k m describes the substrate. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme is suited to. Enzyme Km Binding Affinity.
From www.numerade.com
SOLVEDUnder what conditions can we assume that KM indicates the binding affinity between Enzyme Km Binding Affinity \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. An enzyme's k m describes the substrate. Affinities of enzymes for substrates. Enzyme Km Binding Affinity.
From www.numerade.com
SOLVED Given are the five Km values for the binding of substrates to a particular enzyme. Which Enzyme Km Binding Affinity What it measures, in simple terms, is the affinity an enzyme has for its substrate. Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. \[ e + s \xrightarrow[ ]{k_1}[ es ] \xrightarrow[ ] {k_2} e + p \] the enzyme interacts with the substrate by binding to its. The. Enzyme Km Binding Affinity.
From www.researchgate.net
Binding affinity versus enzyme activity. The enzyme activity of active... Download Scientific Enzyme Km Binding Affinity Two commonly encountered parameters in enzyme kinetics are the michaelis constant (km) and the dissociation constant (kd), both report. Dive into the biochemical reactions that govern enzyme. What it measures, in simple terms, is the affinity an enzyme has for its substrate. Affinities of enzymes for substrates vary considerably, so knowing km helps us to understand how well an enzyme. Enzyme Km Binding Affinity.
From www.researchgate.net
Substratebinding affinity curves for chloramphenicol binding to MdtM... Download Scientific Enzyme Km Binding Affinity The km is a measure of the binding affinity of an enzyme for its ligand (substrate or cofactor) and is defined as the concentration of ligand. The nmt1 example highlights the crucial role of binding affinity in determining the substrate specificity of enzymes, which in contrast to the traditionally. Affinities of enzymes for substrates vary considerably, so knowing km helps. Enzyme Km Binding Affinity.