Standard Enthalpy Of Formation Co . Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. At least two other estimates of the activation. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition.
from www.chegg.com
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. At least two other estimates of the activation. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.
Solved Use a standard enthalpies of formation table to
Standard Enthalpy Of Formation Co The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. At least two other estimates of the activation. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen.
From mungfali.com
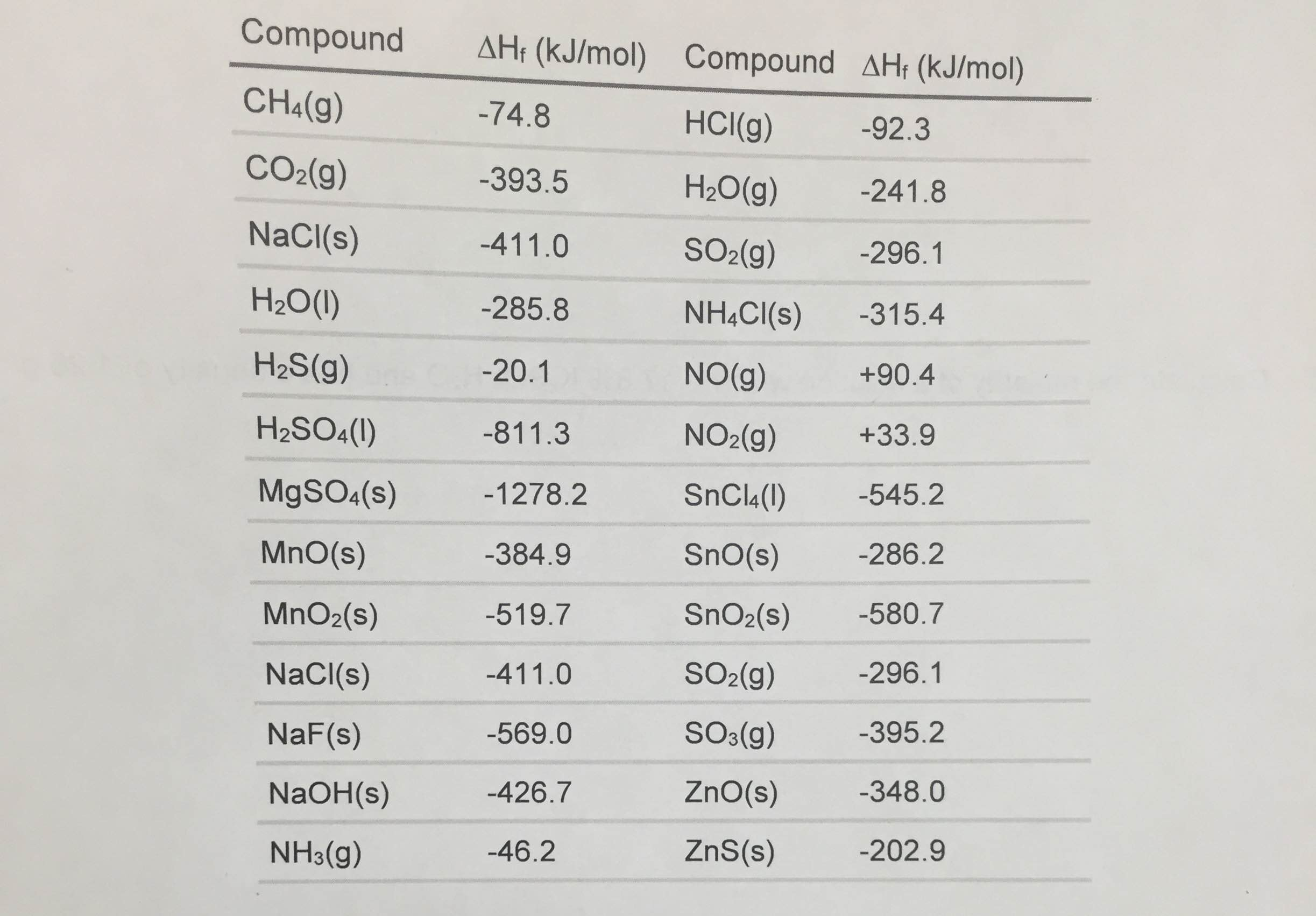
Enthalpies Of Formation Table Standard Enthalpy Of Formation Co 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation of any element in its standard state is zero by definition. At least. Standard Enthalpy Of Formation Co.
From slideplayer.com
Chapter 4 Thermochemistry. ppt download Standard Enthalpy Of Formation Co The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. At least two other estimates of the activation. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. The standard enthalpy of formation of any. Standard Enthalpy Of Formation Co.
From www.youtube.com
Enthalpy of combustion of carbon to CO2 is 393.5KJ mol1. Calculate the heat released upon Standard Enthalpy Of Formation Co At least two other estimates of the activation. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation Co.
From www.numerade.com
SOLVED 2 Using standard heats (8)ZHE of formation, co(e) calculatc the standard enthalpy change Standard Enthalpy Of Formation Co 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. At least two other estimates of the activation. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Enthalpy Of Formation Co.
From www.youtube.com
41524992Given standard enthalpy of formation of `CO(110 \"KJ mol\"^(1))` and `CO_(2)(394 \"KJ Standard Enthalpy Of Formation Co For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. The standard enthalpy of formation of any element in its standard state is zero by definition. Definition and explanation of the terms standard state and standard. Standard Enthalpy Of Formation Co.
From chemistryguru.com.sg
Compare Enthalpy Change of Formation of CO and CO2 Standard Enthalpy Of Formation Co The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of. Standard Enthalpy Of Formation Co.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID3196359 Standard Enthalpy Of Formation Co 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Enthalpy Of Formation Co.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Co Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition.. Standard Enthalpy Of Formation Co.
From cevrpozx.blob.core.windows.net
Standard Heat Of Formation Co at Tamara Schultz blog Standard Enthalpy Of Formation Co Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this. Standard Enthalpy Of Formation Co.
From byjus.com
21. At 300 K standard enthalpy of formation of C6H5COOH(s), CO2(g), and H2O(l) are 408, 393, and Standard Enthalpy Of Formation Co The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. At least two other estimates of the activation. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation of any. Standard Enthalpy Of Formation Co.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Co For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows. Standard Enthalpy Of Formation Co.
From byjus.com
66 Enthalpies of formation of co(g),CO2(g),N2O(g)and N2O4(g) are 110, 398, 81, 97 KJ/lol Standard Enthalpy Of Formation Co The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. At least two other estimates of the activation. 17 rows selected atct [1,. Standard Enthalpy Of Formation Co.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Co 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. At least two other estimates of the activation. The standard enthalpy of formation of any element. Standard Enthalpy Of Formation Co.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Co Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation Co.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Co The standard enthalpy of formation of any element in its standard state is zero by definition. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy. Standard Enthalpy Of Formation Co.
From www.numerade.com
⏩SOLVEDHow can the standard enthalpy of formation of CO(g) be… Numerade Standard Enthalpy Of Formation Co 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118. Standard Enthalpy Of Formation Co.
From www.vrogue.co
Standard Enthalpy Of Reaction Units Slide Share vrogue.co Standard Enthalpy Of Formation Co 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. At least two other estimates of the activation. Definition and explanation of the terms standard. Standard Enthalpy Of Formation Co.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube Standard Enthalpy Of Formation Co For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. At least two other estimates of the activation. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation Co.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation, free download ID2964088 Standard Enthalpy Of Formation Co The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation Co.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Co Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Enthalpy Of Formation Co.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Co Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Formation Co.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation Co 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Of Formation Co.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Enthalpy Of Formation Co The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these. Standard Enthalpy Of Formation Co.
From www.solutionspile.com
[Solved] Using the standard enthalpies of formation, what Standard Enthalpy Of Formation Co At least two other estimates of the activation. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Enthalpy Of Formation Co.
From chemistryguru.com.sg
Compare Enthalpy Change of Formation of CO and CO2 Standard Enthalpy Of Formation Co The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Enthalpy Of Formation Co.
From www.toppr.com
The standard enthalpies of formation of CO2(g), H2O(l) and glucose (s) at 25^∘C are 400 kJ/mol Standard Enthalpy Of Formation Co For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. At least two other estimates. Standard Enthalpy Of Formation Co.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation Co For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. The standard enthalpy. Standard Enthalpy Of Formation Co.
From chemistryguru.com.sg
Compare Enthalpy Change of Formation of CO and CO2 Standard Enthalpy Of Formation Co 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. At least two other estimates of the activation. 17 rows selected atct [1, 2] enthalpy of. Standard Enthalpy Of Formation Co.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Chemistry 2e 5.3 YouTube Standard Enthalpy Of Formation Co The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy. Standard Enthalpy Of Formation Co.
From slidetodoc.com
STANDARD MOLAR ENTHALPY OF FORMATION Enthalpy change when Standard Enthalpy Of Formation Co 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. At least two other estimates of the activation. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Formation Co.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of CH3OH(I) from the following data CH3OH Standard Enthalpy Of Formation Co Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation. Standard Enthalpy Of Formation Co.
From chemistryguru.com.sg
Energy Cycle for Enthalpy Change of Formation Standard Enthalpy Of Formation Co The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation Co.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Enthalpy Of Formation Co At least two other estimates of the activation. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen.. Standard Enthalpy Of Formation Co.
From chemistryguru.com.sg
Compare Enthalpy Change of Formation of CO and CO2 Standard Enthalpy Of Formation Co The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy. Standard Enthalpy Of Formation Co.
From www.numerade.com
SOLVEDFrom the enthalpy of formation for CO2 and the following information, calculate the Standard Enthalpy Of Formation Co 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 17 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. The standard enthalpy of formation of any element in its standard state is zero by definition.. Standard Enthalpy Of Formation Co.