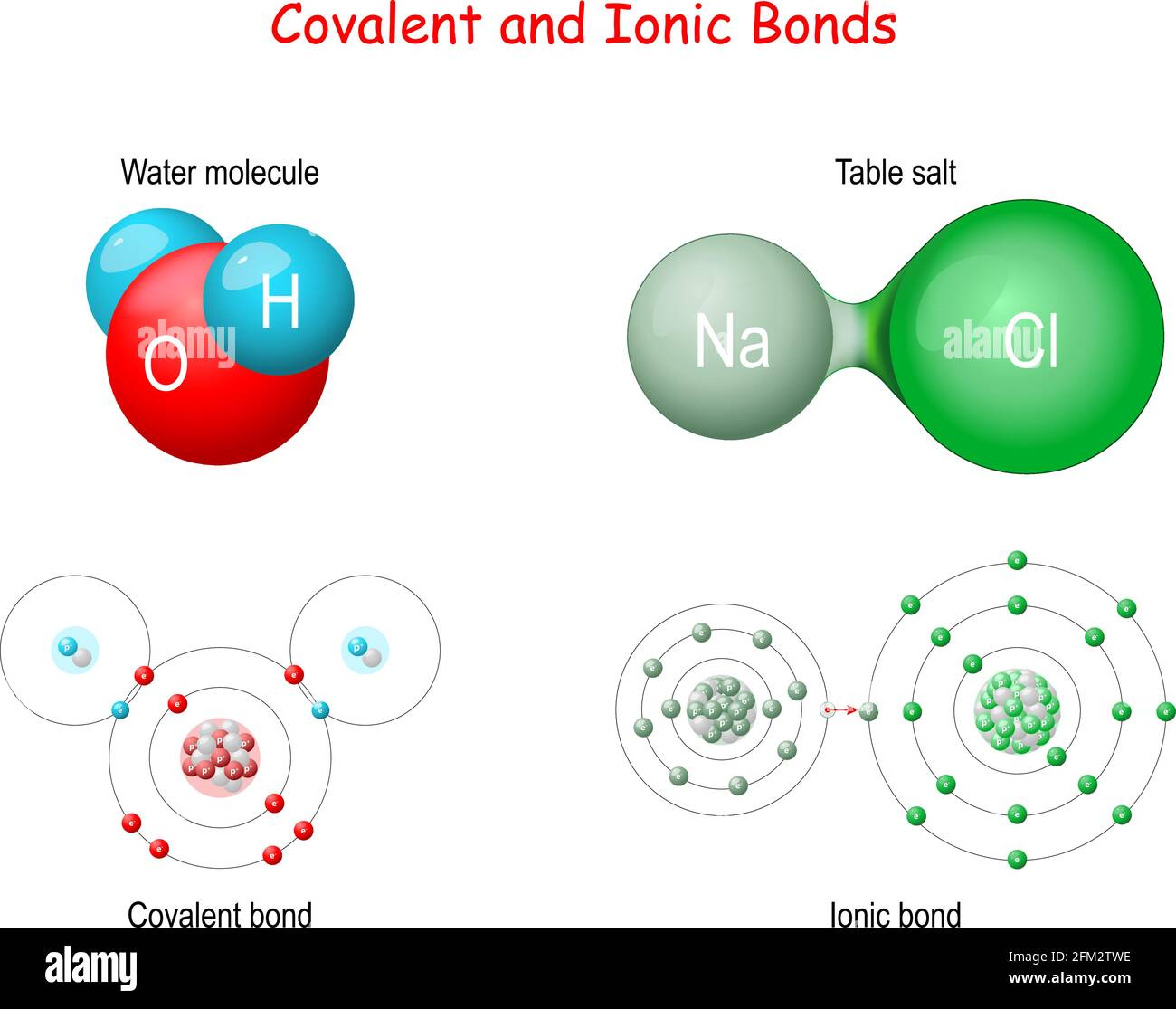
Lead Ii Hydroxide Ionic Or Covalent . We begin with binary ionic compounds, which contain only two elements. The two main types of chemical bonds are ionic and covalent bonds. Thus its oxidation charge would. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. The only pure covalent bonds occur between identical atoms.
from www.alamy.com
The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. We begin with binary ionic compounds, which contain only two elements. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The two main types of chemical bonds are ionic and covalent bonds. The only pure covalent bonds occur between identical atoms. Thus its oxidation charge would.
Ionic bond hires stock photography and images Alamy
Lead Ii Hydroxide Ionic Or Covalent The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. The two main types of chemical bonds are ionic and covalent bonds. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. We begin with binary ionic compounds, which contain only two elements. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. Thus its oxidation charge would. The only pure covalent bonds occur between identical atoms.
From sciencelab.co.ke
Lead Hydroxide Sciencelab limited Lead Ii Hydroxide Ionic Or Covalent We begin with binary ionic compounds, which contain only two elements. The two main types of chemical bonds are ionic and covalent bonds. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The pb^(2+). Lead Ii Hydroxide Ionic Or Covalent.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Lead Ii Hydroxide Ionic Or Covalent We begin with binary ionic compounds, which contain only two elements. The only pure covalent bonds occur between identical atoms. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. Thus its oxidation charge would. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons. Lead Ii Hydroxide Ionic Or Covalent.
From www.chegg.com
Solved What is the solubility of lead(II) hydroxide, Lead Ii Hydroxide Ionic Or Covalent We begin with binary ionic compounds, which contain only two elements. The only pure covalent bonds occur between identical atoms. The two main types of chemical bonds are ionic and covalent bonds. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. Learn. Lead Ii Hydroxide Ionic Or Covalent.
From www.slideserve.com
PPT Review of Atomic Model PowerPoint Presentation, free download Lead Ii Hydroxide Ionic Or Covalent Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. Thus its oxidation charge would. We begin with binary ionic compounds, which contain only two elements. The two main types of chemical bonds are ionic and covalent bonds. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and. Lead Ii Hydroxide Ionic Or Covalent.
From wayneferscarlson.blogspot.com
Is Lead Nitrate Ionic or Covalent Lead Ii Hydroxide Ionic Or Covalent Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The only pure covalent bonds occur between identical atoms. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. The two main types of chemical bonds are ionic and covalent bonds. An ionic bond. Lead Ii Hydroxide Ionic Or Covalent.
From www.sciencephoto.com
Lead (II) hydroxide precipitate, 3 of 3 Stock Image C036/3123 Lead Ii Hydroxide Ionic Or Covalent Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. Thus its oxidation charge would. The two main types of chemical bonds are ionic and covalent bonds. We begin with binary ionic compounds, which. Lead Ii Hydroxide Ionic Or Covalent.
From www.youtube.com
Lead(II) oxide YouTube Lead Ii Hydroxide Ionic Or Covalent The two main types of chemical bonds are ionic and covalent bonds. The only pure covalent bonds occur between identical atoms. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond. Lead Ii Hydroxide Ionic Or Covalent.
From www.slideserve.com
PPT Ionic Charges PowerPoint Presentation, free download ID5125917 Lead Ii Hydroxide Ionic Or Covalent An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The two main types of chemical bonds are ionic and covalent bonds. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. We begin with. Lead Ii Hydroxide Ionic Or Covalent.
From www.numerade.com
SOLVED Mixed Ionic/Covalent Compound Naming For each of the following Lead Ii Hydroxide Ionic Or Covalent An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. Thus its oxidation charge would. We begin with binary ionic compounds, which contain only two. Lead Ii Hydroxide Ionic Or Covalent.
From slideplayer.com
IONIC COMPOUNDS. ppt download Lead Ii Hydroxide Ionic Or Covalent We begin with binary ionic compounds, which contain only two elements. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The two main types of chemical bonds are ionic and covalent bonds. The only pure covalent bonds occur between identical atoms. The pb^(2+) ion has an ionic charge of 2+, meaning. Lead Ii Hydroxide Ionic Or Covalent.
From www.youtube.com
OH Lewis Structure How to Draw the Lewis Dot Structure for the Lead Ii Hydroxide Ionic Or Covalent The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between. Lead Ii Hydroxide Ionic Or Covalent.
From www.numerade.com
SOLVED The net ionic equation for the reaction of aqueous sodium Lead Ii Hydroxide Ionic Or Covalent We begin with binary ionic compounds, which contain only two elements. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. Thus its oxidation charge would. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the. Lead Ii Hydroxide Ionic Or Covalent.
From wayneferscarlson.blogspot.com
Is Lead Nitrate Ionic or Covalent Lead Ii Hydroxide Ionic Or Covalent The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. Thus its oxidation charge would. The two main types of chemical bonds are ionic and covalent bonds. We begin with binary ionic compounds, which contain only two elements. Learn how to name ionic compounds, molecular compounds, and acids based on the rules. Lead Ii Hydroxide Ionic Or Covalent.
From www.youtube.com
Is Ba(OH)2 (Barium hydroxide) Ionic or Covalent? YouTube Lead Ii Hydroxide Ionic Or Covalent The only pure covalent bonds occur between identical atoms. The two main types of chemical bonds are ionic and covalent bonds. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. We begin with binary ionic compounds, which contain only two elements. An ionic bond essentially donates an electron to the other. Lead Ii Hydroxide Ionic Or Covalent.
From www.currentschoolnews.com
Major Differences Between Ionic and Covalent Compounds People Ignore Lead Ii Hydroxide Ionic Or Covalent Thus its oxidation charge would. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. We begin with binary ionic compounds, which contain only two elements. The only pure covalent bonds occur between identical atoms. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than. Lead Ii Hydroxide Ionic Or Covalent.
From wayneferscarlson.blogspot.com
Is Lead Nitrate Ionic or Covalent Lead Ii Hydroxide Ionic Or Covalent An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses. Lead Ii Hydroxide Ionic Or Covalent.
From www.youtube.com
Is Ca(OH)2 Ionic or Covalent/Molecular? YouTube Lead Ii Hydroxide Ionic Or Covalent The only pure covalent bonds occur between identical atoms. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. The two main types of chemical bonds are ionic and covalent bonds. We begin with binary ionic compounds, which contain only two elements. The procedure for naming such compounds is outlined in figure. Lead Ii Hydroxide Ionic Or Covalent.
From www.alamy.com
Ionic bond hires stock photography and images Alamy Lead Ii Hydroxide Ionic Or Covalent The two main types of chemical bonds are ionic and covalent bonds. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. We begin with binary ionic compounds, which contain only two elements. Learn how to name ionic compounds, molecular compounds, and acids. Lead Ii Hydroxide Ionic Or Covalent.
From www.indiamart.com
Lead (II) Hydroxide Acetate Anhydrous at Rs 880/gram Sugar Of Lead in Lead Ii Hydroxide Ionic Or Covalent Thus its oxidation charge would. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. The only pure covalent bonds occur between identical atoms. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. The two main types of chemical bonds are ionic and covalent. Lead Ii Hydroxide Ionic Or Covalent.
From jeopardylabs.com
Ionic vs Covalent & pH Jeopardy Template Lead Ii Hydroxide Ionic Or Covalent Thus its oxidation charge would. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. We begin with binary ionic compounds, which contain only two elements. The only pure covalent bonds occur between identical atoms. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following.. Lead Ii Hydroxide Ionic Or Covalent.
From www.chegg.com
Solved Write the chemical formula of strontium hydroxide Lead Ii Hydroxide Ionic Or Covalent Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. Thus its oxidation charge would. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. The two. Lead Ii Hydroxide Ionic Or Covalent.
From www.slideshare.net
Naming ioniccovalent Lead Ii Hydroxide Ionic Or Covalent An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. We begin with binary ionic compounds, which contain only two elements. The only pure covalent. Lead Ii Hydroxide Ionic Or Covalent.
From slidesharetrick.blogspot.com
Naoh Ionic Or Covalent slidesharetrick Lead Ii Hydroxide Ionic Or Covalent The only pure covalent bonds occur between identical atoms. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. Thus its oxidation charge would. The. Lead Ii Hydroxide Ionic Or Covalent.
From www.numerade.com
SOLVED Draw the Lewis Dot structure for Aluminum hydroxide Al(OH)3 Lead Ii Hydroxide Ionic Or Covalent The two main types of chemical bonds are ionic and covalent bonds. We begin with binary ionic compounds, which contain only two elements. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. Thus its. Lead Ii Hydroxide Ionic Or Covalent.
From www.numerade.com
SOLVEDDetermine if the following (nonelectrolytes) when placed Lead Ii Hydroxide Ionic Or Covalent An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The only pure covalent bonds occur between identical atoms. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. Thus its oxidation charge would. The. Lead Ii Hydroxide Ionic Or Covalent.
From www.youtube.com
How to Write the Formula for Tin (II) oxide YouTube Lead Ii Hydroxide Ionic Or Covalent An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. We begin with binary ionic compounds, which contain only two elements. The two main types of chemical bonds are ionic and covalent bonds. The only pure covalent bonds occur between identical atoms. Thus. Lead Ii Hydroxide Ionic Or Covalent.
From wayneferscarlson.blogspot.com
Is Lead Nitrate Ionic or Covalent Lead Ii Hydroxide Ionic Or Covalent The only pure covalent bonds occur between identical atoms. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The procedure for naming such compounds. Lead Ii Hydroxide Ionic Or Covalent.
From www.numerade.com
SOLVED Text Name of Compound Chemical Formula Type of compound ionic Lead Ii Hydroxide Ionic Or Covalent An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The only pure covalent bonds occur between identical atoms. We begin with binary ionic compounds, which contain only two elements. Learn how to name ionic compounds, molecular compounds, and acids based on the. Lead Ii Hydroxide Ionic Or Covalent.
From www.numerade.com
SOLVED For each of the following compounds, determine if it is ionic Lead Ii Hydroxide Ionic Or Covalent We begin with binary ionic compounds, which contain only two elements. The two main types of chemical bonds are ionic and covalent bonds. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The pb^(2+) ion has an ionic charge of 2+, meaning. Lead Ii Hydroxide Ionic Or Covalent.
From studylib.net
Ionic and Covalent Worksheet Lead Ii Hydroxide Ionic Or Covalent The two main types of chemical bonds are ionic and covalent bonds. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. We begin with. Lead Ii Hydroxide Ionic Or Covalent.
From www.numerade.com
SOLVED Compound Names To name the following compounds, you must first Lead Ii Hydroxide Ionic Or Covalent Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The only pure covalent bonds occur between identical atoms. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. The two main types of chemical bonds are ionic and covalent bonds. We begin with binary. Lead Ii Hydroxide Ionic Or Covalent.
From slideplayer.com
1.ionic iron (II) chloride 2.ionic lead (II) oxide 3.ionic copper Lead Ii Hydroxide Ionic Or Covalent The only pure covalent bonds occur between identical atoms. Thus its oxidation charge would. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. The pb^(2+) ion has an ionic charge of 2+, meaning that it has 2 fewer electrons than protons. The two main types of chemical bonds are ionic and covalent. Lead Ii Hydroxide Ionic Or Covalent.
From www.youtube.com
How to Draw the Lewis Dot Structure for Pb(OH)2 Lead (II) hydroxide Lead Ii Hydroxide Ionic Or Covalent Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The only pure covalent bonds occur between identical atoms. The procedure for naming such compounds. Lead Ii Hydroxide Ionic Or Covalent.
From www.youtube.com
Is Li2O (Lithium oxide) Ionic or Covalent/Molecular? YouTube Lead Ii Hydroxide Ionic Or Covalent The only pure covalent bonds occur between identical atoms. The two main types of chemical bonds are ionic and covalent bonds. Thus its oxidation charge would. We begin with binary ionic compounds, which contain only two elements. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The procedure for naming such. Lead Ii Hydroxide Ionic Or Covalent.
From slideplayer.com
CHEMICAL BONDING Lewis Dot Structures Ionic an Covalent Bonding ppt Lead Ii Hydroxide Ionic Or Covalent We begin with binary ionic compounds, which contain only two elements. Thus its oxidation charge would. The procedure for naming such compounds is outlined in figure \ (\pageindex {1}\) and uses the following. Learn how to name ionic compounds, molecular compounds, and acids based on the rules of charge neutrality, greek. The only pure covalent bonds occur between identical atoms.. Lead Ii Hydroxide Ionic Or Covalent.