Is A Compound A Mixture Of Elements . Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. A mixture is a material which has two or more. In a compound, elements are. a compound is a pure substance which contains identical molecules with two or more types of atomic core. a compound refers to a chemical combination of two or more elements, where a reaction occurs. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. a compound is a pure substance that is made from more than one element. For example, you cannot physically separate water into hydrogen and oxygen. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). if a substance can be separated into its elements, it is a compound. If a substance is not chemically pure, it is either a. A compound cannot be broken down into its original components by physical methods.
from herbalic79.blogspot.com
a compound is a pure substance which contains identical molecules with two or more types of atomic core. if a substance can be separated into its elements, it is a compound. A compound cannot be broken down into its original components by physical methods. In a compound, elements are. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. A mixture is a material which has two or more. a compound is a pure substance that is made from more than one element. a compound refers to a chemical combination of two or more elements, where a reaction occurs. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. For example, you cannot physically separate water into hydrogen and oxygen.
Element Compound Mixture Diagram Worksheet Herbalic
Is A Compound A Mixture Of Elements Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. For example, you cannot physically separate water into hydrogen and oxygen. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. a compound is a pure substance that is made from more than one element. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). A compound cannot be broken down into its original components by physical methods. In a compound, elements are. If a substance is not chemically pure, it is either a. if a substance can be separated into its elements, it is a compound. A mixture is a material which has two or more. a compound is a pure substance which contains identical molecules with two or more types of atomic core. a compound refers to a chemical combination of two or more elements, where a reaction occurs.
From enginedbscientizes.z21.web.core.windows.net
Mixture Of Elements Diagram Is A Compound A Mixture Of Elements Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. If a substance is not chemically pure, it is either a. if a substance can be separated into its elements, it is a compound. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. a compound is a pure. Is A Compound A Mixture Of Elements.
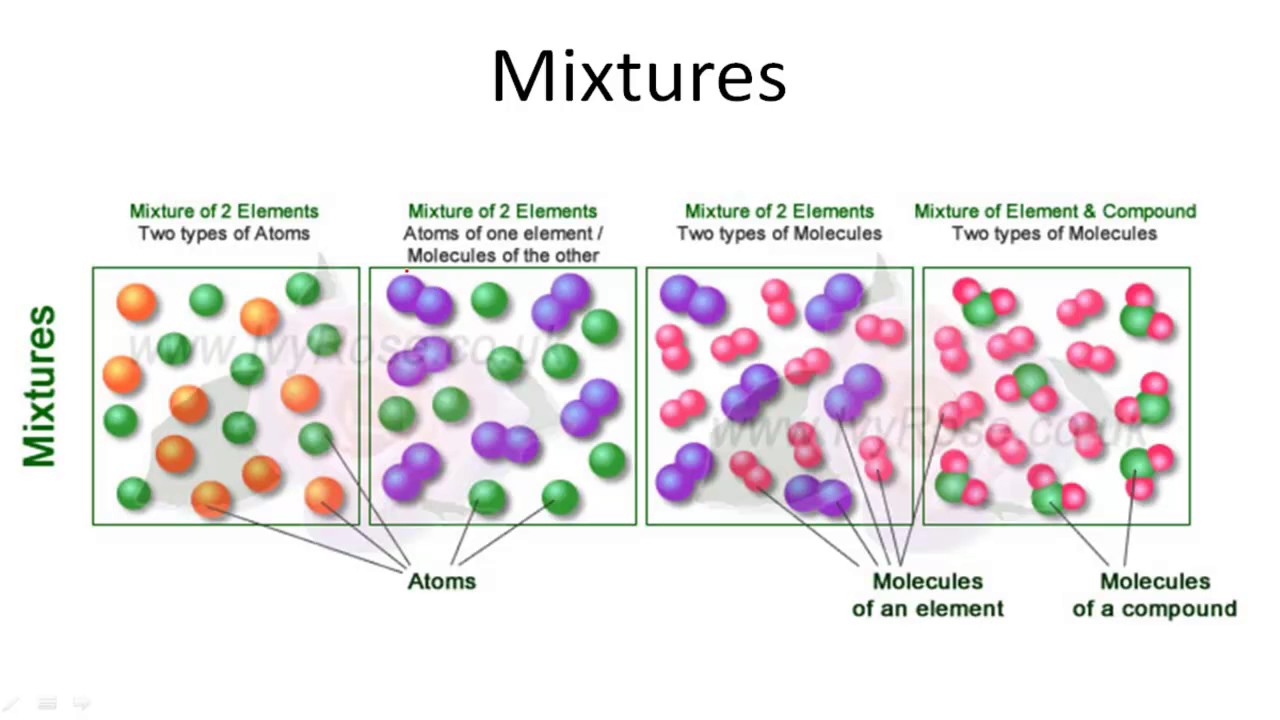
From www.youtube.com
IGCSE Chemistry Elements, Compounds & Mixtures YouTube Is A Compound A Mixture Of Elements if a substance can be separated into its elements, it is a compound. In a compound, elements are. A mixture is a material which has two or more. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). If a. Is A Compound A Mixture Of Elements.
From www.tes.com
Elements, Compound, Mixture (Chemistry) Teaching Resources Is A Compound A Mixture Of Elements if a substance can be separated into its elements, it is a compound. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. a compound refers to a chemical combination of two or more elements, where a reaction occurs. a compound is a pure substance which contains identical molecules with two. Is A Compound A Mixture Of Elements.
From teachingthekid.blogspot.com
Teaching the Kid Elements, Compounds, and Mixtures Is A Compound A Mixture Of Elements For example, you cannot physically separate water into hydrogen and oxygen. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). A compound cannot be broken down into its original components by physical methods. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. If a substance is not chemically pure,. Is A Compound A Mixture Of Elements.
From www.shutterstock.com
Different Between Elements Compounds Mixtures Example Stock Is A Compound A Mixture Of Elements a compound is a pure substance which contains identical molecules with two or more types of atomic core. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. A mixture is a material which has two or more. a compound is a pure substance that is made from more than one element.. Is A Compound A Mixture Of Elements.
From chemistryclinic.co.uk
1.8 Elements, compounds, mixtures Chemistry Is A Compound A Mixture Of Elements a compound is a pure substance that is made from more than one element. In a compound, elements are. if a substance can be separated into its elements, it is a compound. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). If a substance is not chemically pure, it is either. Is A Compound A Mixture Of Elements.
From teachingthekid.blogspot.com
Teaching the Kid Elements, Compounds, and Mixtures Is A Compound A Mixture Of Elements For example, you cannot physically separate water into hydrogen and oxygen. a compound refers to a chemical combination of two or more elements, where a reaction occurs. a compound is a pure substance that is made from more than one element. a compound is a pure substance which contains identical molecules with two or more types of. Is A Compound A Mixture Of Elements.
From www.youtube.com
Pure Substances, Elements, Compounds, Homogenous & Heterogenous Mixture Is A Compound A Mixture Of Elements if a substance can be separated into its elements, it is a compound. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). For example, you cannot physically separate water into hydrogen and oxygen. A compound cannot be broken down into its original components by physical methods. In a compound, elements are. . Is A Compound A Mixture Of Elements.
From www.slideserve.com
PPT Elements, Compounds, Mixtures PowerPoint Presentation, free Is A Compound A Mixture Of Elements A compound cannot be broken down into its original components by physical methods. if a substance can be separated into its elements, it is a compound. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. a compound is a pure substance that is made from more than one element. Microscopic view of a gaseous. Is A Compound A Mixture Of Elements.
From enginedbscientizes.z21.web.core.windows.net
Mixture Of Elements And Compounds Diagram Is A Compound A Mixture Of Elements A mixture is a material which has two or more. a compound refers to a chemical combination of two or more elements, where a reaction occurs. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. a compound is a pure substance which contains identical molecules with two or more types of. Is A Compound A Mixture Of Elements.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples Is A Compound A Mixture Of Elements In a compound, elements are. If a substance is not chemically pure, it is either a. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). if a substance can be separated into its elements, it is a compound. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. . Is A Compound A Mixture Of Elements.
From www.numerade.com
SOLVED 10 POINTS!!!! Classify each as either element compound mixture Is A Compound A Mixture Of Elements a compound is a pure substance that is made from more than one element. if a substance can be separated into its elements, it is a compound. A mixture is a material which has two or more. In a compound, elements are. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements.. Is A Compound A Mixture Of Elements.
From quizlet.com
Elements, Compounds, and Mixtures Diagram Quizlet Is A Compound A Mixture Of Elements Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. In a compound, elements are. a compound refers to a chemical combination of two or more elements, where a reaction occurs. Other examples include sugar, table salt. Is A Compound A Mixture Of Elements.
From stock.adobe.com
illustration of chemistry, Pure Substances and Mixtures, element Is A Compound A Mixture Of Elements a compound refers to a chemical combination of two or more elements, where a reaction occurs. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. a compound is a pure substance that is made from more than one element. a compound is a pure substance which contains identical molecules with. Is A Compound A Mixture Of Elements.
From circuitlibrarycorti.z13.web.core.windows.net
Mixture Of Elements And Compounds Diagram Is A Compound A Mixture Of Elements Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). a compound refers to a chemical combination of two or more elements, where a reaction occurs. For example, you cannot physically separate water into hydrogen and oxygen. a compound is a pure substance that is made from more than one element. . Is A Compound A Mixture Of Elements.
From circuitdiagramalexandra.z5.web.core.windows.net
Element Compound Mixture Diagram Is A Compound A Mixture Of Elements Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. a compound refers to a chemical combination of two or more elements, where a reaction occurs. A mixture is a material which has two or more. In a compound, elements are. If a substance is not chemically pure, it is either a. the compounds water,. Is A Compound A Mixture Of Elements.
From sites.google.com
Unit 4 Elements, Compounds, and Mixtures Ms. Evangelista's MS Science Is A Compound A Mixture Of Elements If a substance is not chemically pure, it is either a. a compound refers to a chemical combination of two or more elements, where a reaction occurs. For example, you cannot physically separate water into hydrogen and oxygen. a compound is a pure substance which contains identical molecules with two or more types of atomic core. Other examples. Is A Compound A Mixture Of Elements.
From worksheetlistwax.z19.web.core.windows.net
Element Compound And Mixture Diagram Is A Compound A Mixture Of Elements a compound is a pure substance that is made from more than one element. In a compound, elements are. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. a compound is a pure substance which contains identical molecules with two or more types of atomic core. a compound refers to a chemical combination. Is A Compound A Mixture Of Elements.
From www.slideserve.com
PPT Elements, Compounds, Mixtures PowerPoint Presentation, free Is A Compound A Mixture Of Elements a compound refers to a chemical combination of two or more elements, where a reaction occurs. A compound cannot be broken down into its original components by physical methods. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. In a compound, elements are. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen). Is A Compound A Mixture Of Elements.
From www.goodscience.com.au
Elements, Compounds and Mixtures Good Science Is A Compound A Mixture Of Elements Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). if a substance can be separated into its elements, it is a compound. a compound is a pure substance which contains identical molecules with two or more types of atomic core. For example, you cannot physically separate water into hydrogen and oxygen.. Is A Compound A Mixture Of Elements.
From chemistry.stackexchange.com
terminology What is the definition of of 'mixture Is A Compound A Mixture Of Elements a compound is a pure substance which contains identical molecules with two or more types of atomic core. a compound refers to a chemical combination of two or more elements, where a reaction occurs. a compound is a pure substance that is made from more than one element. A compound cannot be broken down into its original. Is A Compound A Mixture Of Elements.
From www.slideshare.net
Elements, Compounds, Mixtures Is A Compound A Mixture Of Elements In a compound, elements are. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. if a substance can be separated into its elements, it is a compound. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. A mixture is a material which has two or more. a. Is A Compound A Mixture Of Elements.
From www.slideserve.com
PPT Matter and Change PowerPoint Presentation, free download ID2199820 Is A Compound A Mixture Of Elements a compound refers to a chemical combination of two or more elements, where a reaction occurs. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. In a compound, elements are. if a substance can be. Is A Compound A Mixture Of Elements.
From www.youtube.com
Elements Compounds Mixtures YouTube Is A Compound A Mixture Of Elements If a substance is not chemically pure, it is either a. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. a compound refers to a chemical combination of two or more elements, where a reaction occurs. a compound. Is A Compound A Mixture Of Elements.
From science4geeks.blogspot.com
Science4Geeks Element, Compound and Mixture Is A Compound A Mixture Of Elements A compound cannot be broken down into its original components by physical methods. a compound refers to a chemical combination of two or more elements, where a reaction occurs. a compound is a pure substance which contains identical molecules with two or more types of atomic core. the compounds water, carbon dioxide, and glucose consist of combinations. Is A Compound A Mixture Of Elements.
From wirepartaffectedly.z22.web.core.windows.net
Homogeneous Mixture Particle Diagram Is A Compound A Mixture Of Elements Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). A compound cannot be broken down into its original components by physical methods. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. For example, you cannot physically separate water into hydrogen and oxygen. a compound is. Is A Compound A Mixture Of Elements.
From www.slideserve.com
PPT Elements, Compounds, Mixtures PowerPoint Presentation, free Is A Compound A Mixture Of Elements In a compound, elements are. a compound is a pure substance which contains identical molecules with two or more types of atomic core. A mixture is a material which has two or more. if a substance can be separated into its elements, it is a compound. A compound cannot be broken down into its original components by physical. Is A Compound A Mixture Of Elements.
From enginedbscientizes.z21.web.core.windows.net
Mixture Of Two Elements Diagram Is A Compound A Mixture Of Elements the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. a compound is a pure substance that is made from more than one element. if a substance can be separated into its elements, it is a compound. a compound is a pure substance which contains identical molecules with two or more. Is A Compound A Mixture Of Elements.
From www.youtube.com
Elements, Compounds, Mixtures YouTube Is A Compound A Mixture Of Elements Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. a compound refers to a chemical combination of two or more elements, where a reaction occurs. A mixture is a material which has two or more. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). the compounds water,. Is A Compound A Mixture Of Elements.
From mungfali.com
Element Compound And Mixture Chart Is A Compound A Mixture Of Elements A mixture is a material which has two or more. a compound is a pure substance that is made from more than one element. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). a compound is a pure substance which contains identical molecules with two or more types of atomic core.. Is A Compound A Mixture Of Elements.
From www.pinterest.com
Element, Mixture, Compound Activity Teaching chemistry, Physical Is A Compound A Mixture Of Elements If a substance is not chemically pure, it is either a. a compound refers to a chemical combination of two or more elements, where a reaction occurs. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. a compound is a pure substance that is made from more than one element. a compound is. Is A Compound A Mixture Of Elements.
From herbalic79.blogspot.com
Element Compound Mixture Diagram Worksheet Herbalic Is A Compound A Mixture Of Elements the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. if a substance can be separated into its elements, it is a compound. A mixture is a material which has two or more. a compound is a pure substance which contains identical molecules with two or more types of atomic core. In. Is A Compound A Mixture Of Elements.
From www.tes.com
Elements, compounds and mixtures Teaching Resources Is A Compound A Mixture Of Elements a compound is a pure substance which contains identical molecules with two or more types of atomic core. if a substance can be separated into its elements, it is a compound. Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. If a substance is not chemically pure, it is either a. For example, you. Is A Compound A Mixture Of Elements.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps Is A Compound A Mixture Of Elements if a substance can be separated into its elements, it is a compound. a compound is a pure substance that is made from more than one element. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Other examples include sugar, table salt (nacl), baking soda (nahco3), carbon dioxide, etc. A compound. Is A Compound A Mixture Of Elements.
From manualfixageundeployed.z13.web.core.windows.net
Mixture Of Compounds Diagram Is A Compound A Mixture Of Elements If a substance is not chemically pure, it is either a. A mixture is a material which has two or more. the compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements. A compound cannot be broken down into its original components by physical methods. a compound refers to a chemical combination of two. Is A Compound A Mixture Of Elements.