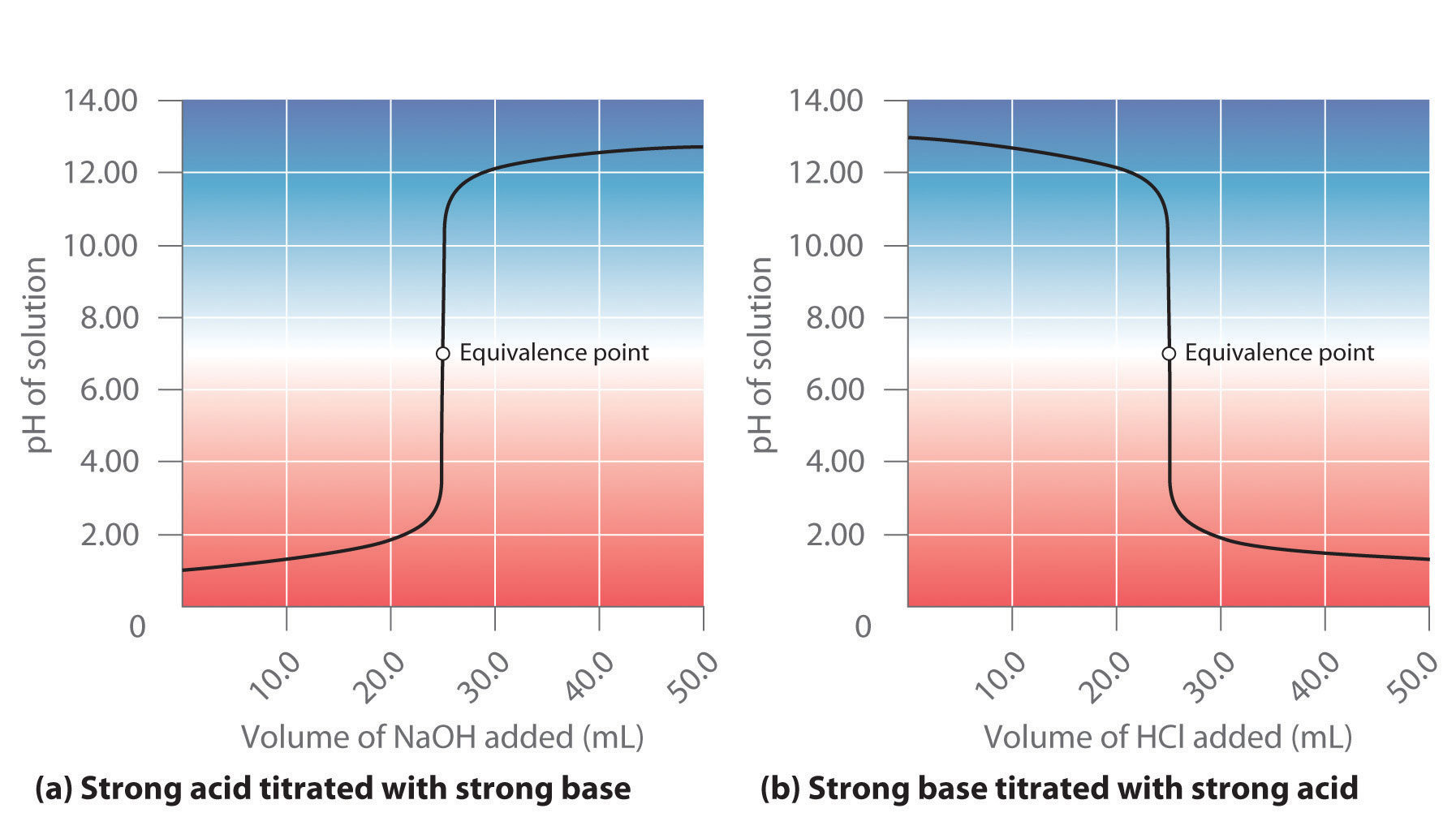
Titration Formula . learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v is volume. Find out the formula, the titration curve, and the types of titrations with examples and references. Find out how to use. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. Learn the principle, formula and types of titration with examples and applications. titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the.
from
At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. Find out the formula, the titration curve, and the types of titrations with examples and references. Learn the principle, formula and types of titration with examples and applications. learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. Find out how to use. titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v is volume.
Titration Formula titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v is volume. titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. Learn the principle, formula and types of titration with examples and applications. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. Find out the formula, the titration curve, and the types of titrations with examples and references. Find out how to use. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Titration Formula Find out how to use. Learn the principle, formula and types of titration with examples and applications. Find out the formula, the titration curve, and the types of titrations with examples and references. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte). Titration Formula.
From
Titration Formula a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Find out how to use. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to. Titration Formula.
From www.vrogue.co
Gcse Chemistry Titration Calculations Teaching Resources www.vrogue.co Titration Formula learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. learn how. Titration Formula.
From
Titration Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Learn the principle, formula and types of titration with examples and applications. learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v. Titration Formula.
From
Titration Formula titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. Find out the formula, the titration curve, and the types of titrations with examples and references. Find out how to use. learn how to measure the concentration of a substance in a solution using titration, a technique. Titration Formula.
From
Titration Formula Learn the principle, formula and types of titration with examples and applications. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. learn how to. Titration Formula.
From
Titration Formula a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v is. Titration Formula.
From
Titration Formula learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. a titration. Titration Formula.
From mappingmemories.ca
emulsión liberal comienzo titration experiment calculations Titration Formula learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v is volume. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Titration Formula.
From exonhrnmo.blob.core.windows.net
Titration Formula Ratio at Sarah Campbell blog Titration Formula Find out the formula, the titration curve, and the types of titrations with examples and references. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. learn how to predict the ph and solution composition at. Titration Formula.
From
Titration Formula learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. Find out the formula, the titration curve, and the types of titrations with examples and references. a titration is a volumetric technique in which a solution. Titration Formula.
From
Titration Formula learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. Find out how to use. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. titration is a technique to determine the concentration of an unknown solution. Titration Formula.
From
Titration Formula learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v is volume. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Titration Formula.
From www.slideserve.com
PPT Unit 13 PowerPoint Presentation, free download ID7012000 Titration Formula learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. At the equivalence point in. Titration Formula.
From www.hanlin.com
IB DP Chemistry SL复习笔记1.2.9 Titrations翰林国际教育 Titration Formula titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. Find out the formula, the. Titration Formula.
From
Titration Formula Find out the formula, the titration curve, and the types of titrations with examples and references. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. titration is a technique to determine the concentration of an. Titration Formula.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Formula learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. . Titration Formula.
From collegedunia.com
Titration Formula Types, Process & Acid Base Indicators Titration Formula Find out how to use. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. a titration is a volumetric technique. Titration Formula.
From
Titration Formula Learn the principle, formula and types of titration with examples and applications. Find out the formula, the titration curve, and the types of titrations with examples and references. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. titration is a technique to. Titration Formula.
From
Titration Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. Find out the formula, the titration curve, and the types of titrations with examples and references.. Titration Formula.
From
Titration Formula learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte). Titration Formula.
From
Titration Formula Learn the principle, formula and types of titration with examples and applications. Find out how to use. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte until an endpoint is reached. a titration is a volumetric technique in which a. Titration Formula.
From
Titration Formula Learn the principle, formula and types of titration with examples and applications. Find out how to use. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. At the equivalence point in a neutralization, the moles of acid are equal to the moles of. Titration Formula.
From
Titration Formula titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. Find out the formula, the titration curve, and the types of titrations with examples and references. learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the. Titration Formula.
From
Titration Formula Learn the principle, formula and types of titration with examples and applications. learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. Find out. Titration Formula.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Formula titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. Find out how to use. learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v is volume. . Titration Formula.
From
Titration Formula titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. Find out how to use. Learn the principle, formula and types of titration with examples and applications. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. learn how. Titration Formula.
From dxompffry.blob.core.windows.net
Titration Formula Class 12 at Donald Rios blog Titration Formula Learn the principle, formula and types of titration with examples and applications. learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b,. Titration Formula.
From
Titration Formula learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v is volume. learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. titration is a. Titration Formula.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration Formula titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. Learn the principle, formula and types of titration with examples and applications. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases.. Titration Formula.
From www.youtube.com
Chemistry Concepts Dilution and Titration Calculations.mp4 YouTube Titration Formula a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Find out how to use. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. learn how to calculate the molarity of. Titration Formula.
From
Titration Formula learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. learn how to predict the ph and solution composition at various points in a titration using different strategies for strong and weak acids and bases. learn how to calculate titrations using the equation nh+. Titration Formula.
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog Titration Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh− ⋅ m b ⋅ v b, where m is molarity and v is volume. Find out how to use. learn how to predict. Titration Formula.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Titration Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. learn how to calculate the molarity of an acid or base using the molarity and volume of the titrant at the equivalence point. learn how to calculate titrations using the equation nh+ ⋅ m a ⋅ v a = noh−. Titration Formula.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Formula titration is a technique to determine the concentration of an unknown solution by adding a solution of known concentration in drops. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a. Titration Formula.