Zinc Electrons Gained Or Lost . Oxidation is the loss of electrons by a molecule, atom, or ion. When one species is oxidized, another is reduced. Reduction is the gain of electrons. One way of remembering which is which is the mnemonic “oil rig”: To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. The other half of the equation involves the hydrogen ions (initially bonded to. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. One part focuses on the loss of electrons, and one part. These two processes always occur together; Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation is loss, reduction is gain.
from periodictable.me
In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: When one species is oxidized, another is reduced. Reduction is the gain of electrons. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. Oxidation is the loss of electrons by a molecule, atom, or ion. These two processes always occur together; The other half of the equation involves the hydrogen ions (initially bonded to. Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. One way of remembering which is which is the mnemonic “oil rig”:
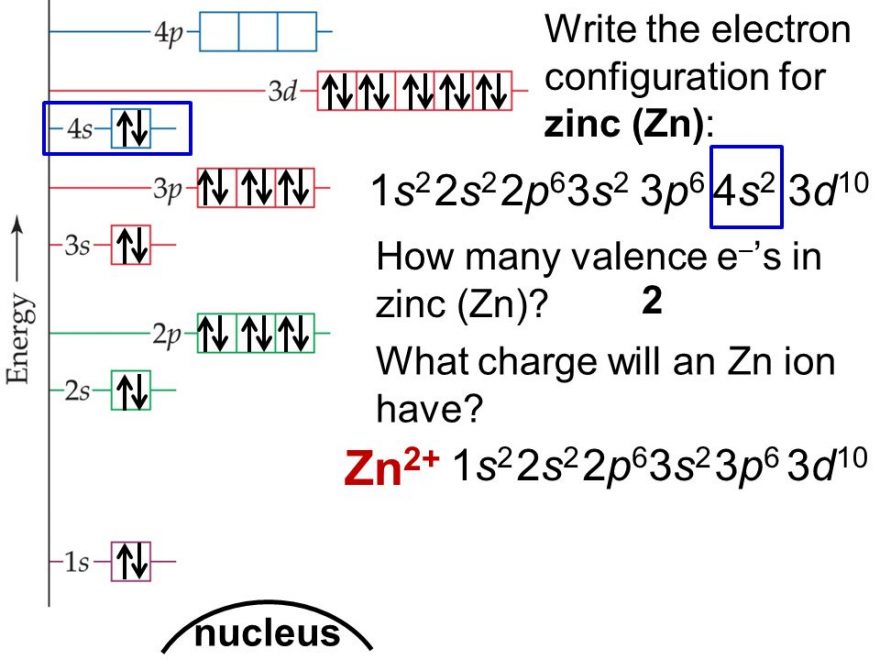
Zinc Electron Configuration (Zn) with Orbital Diagram
Zinc Electrons Gained Or Lost In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. These two processes always occur together; Oxidation is the loss of electrons by a molecule, atom, or ion. When one species is oxidized, another is reduced. One part focuses on the loss of electrons, and one part. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Oxidation is loss, reduction is gain. The other half of the equation involves the hydrogen ions (initially bonded to. Reduction is the gain of electrons. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. One way of remembering which is which is the mnemonic “oil rig”: Since the zinc atom lost electrons, it is an oxidation reaction. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them.
From animalia-life.club
Zinc Electron Configuration Zinc Electrons Gained Or Lost Oxidation is the loss of electrons by a molecule, atom, or ion. Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. One part focuses on the loss of electrons, and one part. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: These. Zinc Electrons Gained Or Lost.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Electrons Gained Or Lost Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. Reduction is the gain of electrons. One way of remembering. Zinc Electrons Gained Or Lost.
From animalia-life.club
Zinc Electron Configuration Zinc Electrons Gained Or Lost One part focuses on the loss of electrons, and one part. Reduction is the gain of electrons. Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation is loss, reduction is gain. Oxidation is the loss of electrons by a molecule, atom, or ion. Oxidation numbers quantify the number of electrons lost or gained by an element during. Zinc Electrons Gained Or Lost.
From slideplayer.com
Chapter 5 Introduction to Reactions in Aqueous Solutions ppt download Zinc Electrons Gained Or Lost In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. These two processes always occur together; Reduction is the gain of electrons. Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation is the loss of electrons by a molecule, atom, or ion. When one. Zinc Electrons Gained Or Lost.
From periodictable.me
Zinc Electron Configuration (Zn) with Orbital Diagram Zinc Electrons Gained Or Lost Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. These two processes always occur together; Oxidation is the loss of electrons by a molecule, atom, or ion. Oxidation is loss,. Zinc Electrons Gained Or Lost.
From www.britannica.com
zinc Properties, Uses, & Facts Britannica Zinc Electrons Gained Or Lost One way of remembering which is which is the mnemonic “oil rig”: Reduction is the gain of electrons. Since the zinc atom lost electrons, it is an oxidation reaction. One part focuses on the loss of electrons, and one part. When one species is oxidized, another is reduced. Oxidation is loss, reduction is gain. To balance a redox equation using. Zinc Electrons Gained Or Lost.
From animalia-life.club
Zinc Electron Configuration Zinc Electrons Gained Or Lost Oxidation is loss, reduction is gain. Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. The other half of the equation involves the hydrogen ions (initially bonded to. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. One part. Zinc Electrons Gained Or Lost.
From slideplayer.com
Reactions in Aqueous Solution ppt download Zinc Electrons Gained Or Lost To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: One way of remembering which is which is the mnemonic “oil rig”: Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation is the loss of electrons by a molecule, atom, or ion. The other half of the equation. Zinc Electrons Gained Or Lost.
From slideplayer.com
Reaction Types. ppt download Zinc Electrons Gained Or Lost Oxidation is loss, reduction is gain. These two processes always occur together; To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: One part focuses on the loss of electrons, and one part. Reduction is the gain of electrons. Oxidation numbers quantify the number of electrons lost or gained by an. Zinc Electrons Gained Or Lost.
From elchoroukhost.net
Periodic Table Zinc Protons Neutrons Electrons Elcho Table Zinc Electrons Gained Or Lost When one species is oxidized, another is reduced. One way of remembering which is which is the mnemonic “oil rig”: Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. Oxidation is loss, reduction is gain. The other half of the equation involves the hydrogen ions (initially bonded. Zinc Electrons Gained Or Lost.
From valenceelectrons.com
How to Write the Electron Configuration for Zinc (Zn)? Zinc Electrons Gained Or Lost Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. The other half of the equation involves the hydrogen ions (initially bonded to. Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation numbers quantify the number of electrons lost or gained by an element during. Zinc Electrons Gained Or Lost.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Electrons Gained Or Lost These two processes always occur together; Reduction is the gain of electrons. Since the zinc atom lost electrons, it is an oxidation reaction. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. One part focuses on the loss of electrons, and one part. One way of remembering. Zinc Electrons Gained Or Lost.
From valenceelectrons.com
How many valence electrons does zinc(Zn) have? Zinc Electrons Gained Or Lost These two processes always occur together; Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. One part focuses on the loss of electrons, and one part. Oxidation is loss, reduction is gain. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: The. Zinc Electrons Gained Or Lost.
From animalia-life.club
Zinc Electron Configuration Zinc Electrons Gained Or Lost When one species is oxidized, another is reduced. One way of remembering which is which is the mnemonic “oil rig”: Oxidation is the loss of electrons by a molecule, atom, or ion. Since the zinc atom lost electrons, it is an oxidation reaction. One part focuses on the loss of electrons, and one part. To balance a redox equation using. Zinc Electrons Gained Or Lost.
From slideplayer.com
Chapter 6 The Periodic Table. ppt download Zinc Electrons Gained Or Lost Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation is the loss of electrons by a molecule, atom, or ion. One part focuses on the loss of electrons, and one part. These two processes always occur together; Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the. Zinc Electrons Gained Or Lost.
From slideplayer.com
Chapter 12 Chemical Bonding by Christopher Hamaker ppt download Zinc Electrons Gained Or Lost Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: One part focuses on the loss of electrons, and one part. When one species is oxidized, another is reduced. Oxygen atoms gain electrons, making. Zinc Electrons Gained Or Lost.
From www.slideshare.net
Understanding The Chemistry Of Atoms to Ions Zinc Electrons Gained Or Lost Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation is loss, reduction is gain. When one species is oxidized, another is reduced. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. Oxidation is the loss of electrons by a molecule, atom, or ion. Reduction. Zinc Electrons Gained Or Lost.
From animalia-life.club
Zinc Electron Configuration Zinc Electrons Gained Or Lost Reduction is the gain of electrons. When one species is oxidized, another is reduced. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Since the zinc atom lost electrons, it is an oxidation reaction. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized,. Zinc Electrons Gained Or Lost.
From www.youtube.com
Determining the number of electrons lost or gained YouTube Zinc Electrons Gained Or Lost To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. These two processes always occur together; Oxygen atoms gain electrons, making it possible for zinc atoms to lose. Zinc Electrons Gained Or Lost.
From slideplayer.com
Electrochemistry Lesson 2 ppt download Zinc Electrons Gained Or Lost To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: These two processes always occur together; The other half of the equation involves the hydrogen ions (initially bonded to. Since the zinc atom lost electrons, it is an oxidation reaction. When one species is oxidized, another is reduced. Reduction is the. Zinc Electrons Gained Or Lost.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Zinc Electrons Gained Or Lost Reduction is the gain of electrons. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. When one species is oxidized, another is reduced. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. To. Zinc Electrons Gained Or Lost.
From studypoinsettia.z4.web.core.windows.net
What Is Zinc Electron Configuration Zinc Electrons Gained Or Lost When one species is oxidized, another is reduced. Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. One part focuses on the loss of electrons, and one part. Oxidation is loss, reduction is gain. Oxidation is the loss of electrons by a molecule, atom, or ion. Reduction. Zinc Electrons Gained Or Lost.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Zinc Electrons Gained Or Lost To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper. Zinc Electrons Gained Or Lost.
From www.slideserve.com
PPT Zinc PowerPoint Presentation, free download ID6832560 Zinc Electrons Gained Or Lost Oxidation is the loss of electrons by a molecule, atom, or ion. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. One part focuses on the loss of electrons, and one part. One way of remembering which is which is the mnemonic “oil rig”: These two. Zinc Electrons Gained Or Lost.
From elchoroukhost.net
Zinc Periodic Table Protons Neutrons Electrons Elcho Table Zinc Electrons Gained Or Lost One way of remembering which is which is the mnemonic “oil rig”: To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Since the zinc atom lost electrons, it is an oxidation reaction. When one species is oxidized, another is reduced. One part focuses on the loss of electrons, and one. Zinc Electrons Gained Or Lost.
From hxeywoprh.blob.core.windows.net
How Many Electrons Does Zinc Lose at Kevin Love blog Zinc Electrons Gained Or Lost Since the zinc atom lost electrons, it is an oxidation reaction. One part focuses on the loss of electrons, and one part. These two processes always occur together; Oxidation is the loss of electrons by a molecule, atom, or ion. Oxidation is loss, reduction is gain. To balance a redox equation using the oxidation state method, we conceptually separate the. Zinc Electrons Gained Or Lost.
From slideplayer.com
Electrochemistry Movement of Electrons Chemistry of Metal Reactions Zinc Electrons Gained Or Lost The other half of the equation involves the hydrogen ions (initially bonded to. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. These two processes always occur together; To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two. Zinc Electrons Gained Or Lost.
From slideplayer.com
Elements and Ions. ppt download Zinc Electrons Gained Or Lost To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: One way of remembering which is which is the mnemonic “oil rig”: Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. One part focuses on the loss of electrons, and one part. In. Zinc Electrons Gained Or Lost.
From www.youtube.com
How many valence electrons are in zinc? YouTube Zinc Electrons Gained Or Lost To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: These two processes always occur together; In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation. Zinc Electrons Gained Or Lost.
From slideplayer.com
Electrochemistry Lesson 1 Introduction. ppt download Zinc Electrons Gained Or Lost Since the zinc atom lost electrons, it is an oxidation reaction. One way of remembering which is which is the mnemonic “oil rig”: When one species is oxidized, another is reduced. The other half of the equation involves the hydrogen ions (initially bonded to. These two processes always occur together; Oxygen atoms gain electrons, making it possible for zinc atoms. Zinc Electrons Gained Or Lost.
From www.nuclear-power.com
Zinc Electron Affinity Electronegativity Ionization Energy of Zinc Electrons Gained Or Lost One part focuses on the loss of electrons, and one part. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: When one species is oxidized, another is reduced. Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. Reduction is the gain of. Zinc Electrons Gained Or Lost.
From exofdpbhd.blob.core.windows.net
Zinc Electrons Level at Armanda Rael blog Zinc Electrons Gained Or Lost The other half of the equation involves the hydrogen ions (initially bonded to. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: These two processes always occur together; One part focuses on the loss of electrons, and one part. Oxidation is the loss of electrons by a molecule, atom, or. Zinc Electrons Gained Or Lost.
From narodnatribuna.info
Zinc Atomic Structure Zinc Electrons Gained Or Lost To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Oxygen atoms gain electrons, making it possible for zinc atoms to lose electrons and be oxidized, so o2 is the oxidizing agent. These two processes always occur together; Reduction is the gain of electrons. When one species is oxidized, another is. Zinc Electrons Gained Or Lost.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Zinc Electrons Gained Or Lost Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. Oxidation is loss, reduction is gain. The other half of the equation involves the hydrogen ions (initially bonded to. Since the zinc atom lost electrons, it is an oxidation reaction. When one species is oxidized, another is reduced. One way of remembering which. Zinc Electrons Gained Or Lost.
From slidetodoc.com
Electrochemistry Lesson 3 Galvanic Cells Review l Zn Zinc Electrons Gained Or Lost Oxidation numbers quantify the number of electrons lost or gained by an element during a chemical reaction. When one species is oxidized, another is reduced. These two processes always occur together; One way of remembering which is which is the mnemonic “oil rig”: Oxidation is the loss of electrons by a molecule, atom, or ion. In the case of a. Zinc Electrons Gained Or Lost.