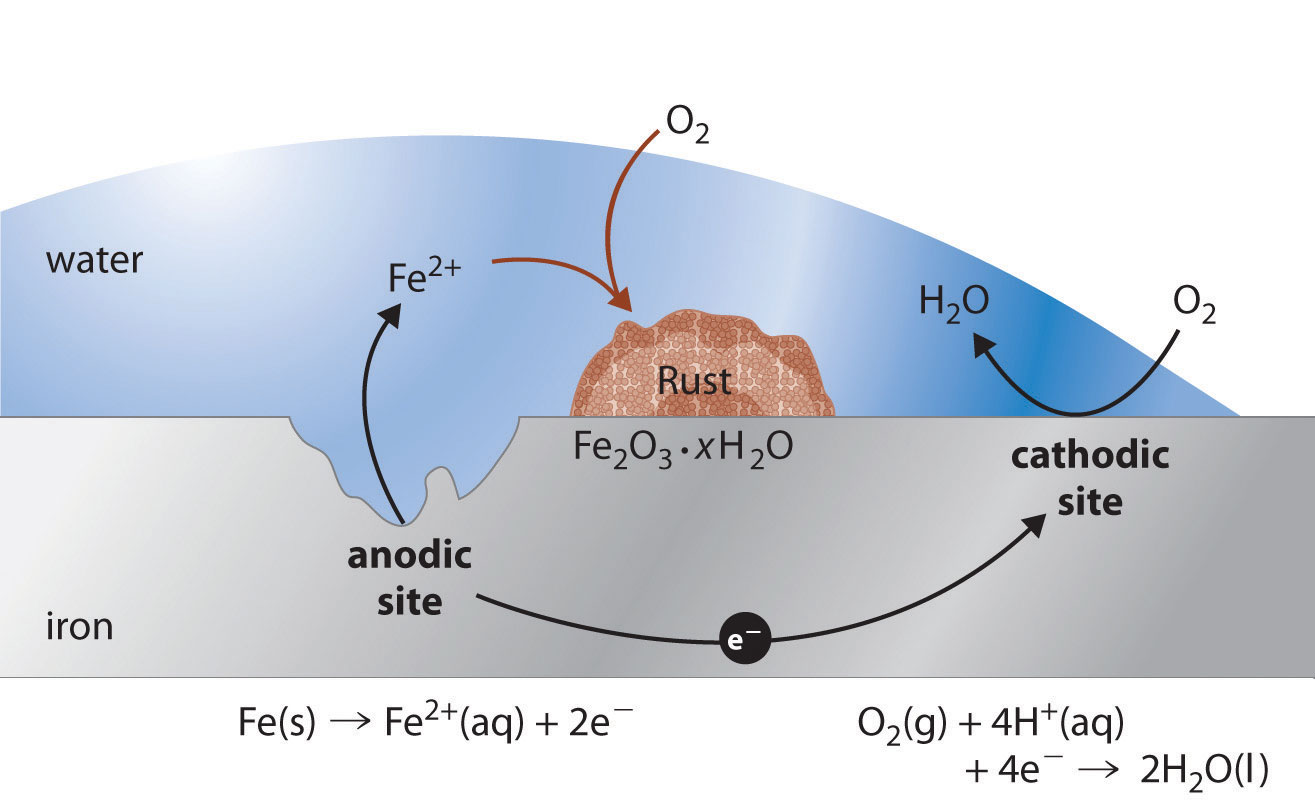
Stainless Steel And Aluminum Reaction . Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: There can be several reasons behind. Stainless steel (active) + aluminum. Below is a galvanic reaction chart for dissimilar metals. Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Stainless steel acts as the cathode, and aluminum acts as an anode. Find out the methods and materials to prevent galvanic. This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid.
from chem.libretexts.org
Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); Find out the methods and materials to prevent galvanic. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Stainless steel acts as the cathode, and aluminum acts as an anode. This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. There can be several reasons behind. Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid.
Chapter 19.6 Corrosion Chemistry LibreTexts
Stainless Steel And Aluminum Reaction Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid. Find out the methods and materials to prevent galvanic. Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. There can be several reasons behind. Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. Stainless steel acts as the cathode, and aluminum acts as an anode. 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. Stainless steel (active) + aluminum. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Below is a galvanic reaction chart for dissimilar metals.
From www.museoinclusivo.com
Does Aluminum React with Stainless Steel? A Comprehensive Look Stainless Steel And Aluminum Reaction Stainless steel (active) + aluminum. Stainless steel acts as the cathode, and aluminum acts as an anode. Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid. There can be several reasons behind. Below is a galvanic reaction chart for dissimilar metals. If the surface area of aluminum is larger. Stainless Steel And Aluminum Reaction.
From mungfali.com
Galvanic Corrosion Diagram Stainless Steel And Aluminum Reaction 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. Below is a galvanic reaction chart for dissimilar metals.. Stainless Steel And Aluminum Reaction.
From metalprofy.com
The Differences Between Stainless Steel and Aluminum MetalProfy Stainless Steel And Aluminum Reaction Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid. Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); This occurs because aluminum is more chemically reactive than. Stainless Steel And Aluminum Reaction.
From www.pinterest.com
Electrochemistry Electrochemistry, Chemistry lessons, Chemistry Stainless Steel And Aluminum Reaction Stainless steel acts as the cathode, and aluminum acts as an anode. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. Below is a galvanic reaction chart for dissimilar metals. If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. Aluminum negatively reacts to. Stainless Steel And Aluminum Reaction.
From steelfabservices.com.au
How To Protect From Steel Corrosion Steel Fab Services Stainless Steel And Aluminum Reaction 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Below is a galvanic reaction chart for dissimilar metals.. Stainless Steel And Aluminum Reaction.
From www.youtube.com
Stainless Steel vs Aluminium In 60 Seconds YouTube Stainless Steel And Aluminum Reaction This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. There can be several reasons behind. Stainless steel acts as the cathode, and aluminum acts as an anode. Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Learn why aluminum. Stainless Steel And Aluminum Reaction.
From aj-racks.com
Aluminum Vs. Steel An Infographic A & J Manufacturing Co. Stainless Steel And Aluminum Reaction Stainless steel (active) + aluminum. 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely. Stainless Steel And Aluminum Reaction.
From chem.libretexts.org
Chapter 19.6 Corrosion Chemistry LibreTexts Stainless Steel And Aluminum Reaction Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Stainless steel acts as the cathode, and aluminum acts as an anode. There can be several reasons behind. Find out the methods and materials to prevent galvanic. Learn why. Stainless Steel And Aluminum Reaction.
From www.jlconline.com
Separating Galvanic Metals JLC Online Stainless Steel And Aluminum Reaction Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); Stainless steel acts as the cathode, and aluminum acts as an anode. Stainless steel (active) + aluminum. Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Learn how stainless steel and aluminium can corrode each other when in. Stainless Steel And Aluminum Reaction.
From blog.thepipingmart.com
Comparing the Strength of Aluminium vs Steel Stainless Steel And Aluminum Reaction Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. Below. Stainless Steel And Aluminum Reaction.
From www.eng-tips.com
Galvanic Corrosion Between Stainless Steel and Aluminium Metal and Stainless Steel And Aluminum Reaction Stainless steel acts as the cathode, and aluminum acts as an anode. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. There can be several reasons behind. Below is a galvanic reaction chart for dissimilar metals. Find out the methods and materials to prevent galvanic. Aluminum negatively reacts to stainless steel but with a. Stainless Steel And Aluminum Reaction.
From www.practicalmachinist.com
Cast aluminum/stainless bolt galvanic corrosion. Best chemical or Stainless Steel And Aluminum Reaction There can be several reasons behind. Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Stainless. Stainless Steel And Aluminum Reaction.
From www.monarchmetal.com
Galvanic Reaction Metals Responsible for Corrosion Stainless Steel And Aluminum Reaction Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum. Stainless Steel And Aluminum Reaction.
From finemetalrooftech.com
Combining roof metals to avoid galvanic reaction Fine Metal Roof Tech Stainless Steel And Aluminum Reaction Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. Stainless steel and aluminum are both widely used metals, but. Stainless Steel And Aluminum Reaction.
From www.museoinclusivo.com
Does Stainless Steel React with Aluminum? Exploring the Interaction Stainless Steel And Aluminum Reaction Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); Stainless steel acts as the cathode, and aluminum acts as an anode. Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by. Stainless Steel And Aluminum Reaction.
From www.museoinclusivo.com
Does Aluminum React with Stainless Steel? A Comprehensive Look Stainless Steel And Aluminum Reaction Below is a galvanic reaction chart for dissimilar metals. Stainless steel acts as the cathode, and aluminum acts as an anode. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. There can be several reasons behind.. Stainless Steel And Aluminum Reaction.
From www.ssina.com
Galvanic Corrosion SSINA Stainless Steel And Aluminum Reaction Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid. This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. This. Stainless Steel And Aluminum Reaction.
From www.intermarineboats.com
Galvanic Corrosion Stainless Steel And Aluminum Reaction If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. Below is a galvanic reaction chart for dissimilar metals. 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: Find out the methods and materials to prevent galvanic. Stainless steel (active) + aluminum. There. Stainless Steel And Aluminum Reaction.
From www.eng-tips.com
Galvanic Corrosion Between Stainless Steel and Aluminium Metal and Stainless Steel And Aluminum Reaction Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. There can be several reasons behind. Below is a galvanic reaction chart for. Stainless Steel And Aluminum Reaction.
From captaincorrosion.com
Galvanic Corrosion Simulator Captain Corrosion Stainless Steel And Aluminum Reaction Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); Stainless steel acts as the cathode, and aluminum acts as an anode. Stainless steel (active) + aluminum. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. Find out the methods and materials to prevent galvanic. Below is a galvanic reaction chart. Stainless Steel And Aluminum Reaction.
From www.pinterest.at
Galvanic Corrosion aluminium steel metal brass bolt fixture Stainless Steel And Aluminum Reaction Stainless steel (active) + aluminum. If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: There can be. Stainless Steel And Aluminum Reaction.
From www.mcfeelys.com
Mixing Metals in Fasteners Stainless Steel And Aluminum Reaction Find out the methods and materials to prevent galvanic. Stainless steel (active) + aluminum. 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to. Stainless Steel And Aluminum Reaction.
From mavink.com
Galvanic Corrosion Aluminum Stainless Steel Stainless Steel And Aluminum Reaction This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. Below is a galvanic reaction chart for dissimilar metals. Stainless steel acts as the cathode, and aluminum acts as an anode. There can be several reasons behind. Stainless steel and aluminum can be used together but in most cases,. Stainless Steel And Aluminum Reaction.
From www.wieland-diversified.com
The Difference Between Aluminum and Stainless Steel Stainless Steel And Aluminum Reaction There can be several reasons behind. 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. Stainless steel and. Stainless Steel And Aluminum Reaction.
From www.researchgate.net
Does chemical reaction occur between steel and aluminum... Stainless Steel And Aluminum Reaction This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to moisture or certain electrolytes. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Find out the methods and materials to. Stainless Steel And Aluminum Reaction.
From www.eng-tips.com
Galvanic Corrosion Between Stainless Steel and Aluminium Metal and Stainless Steel And Aluminum Reaction Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. There can be several reasons behind. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Learn why aluminum and stainless steel. Stainless Steel And Aluminum Reaction.
From www.scribd.com
Galvanic Corrosion PDF Corrosion Stainless Steel Stainless Steel And Aluminum Reaction Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Below is a galvanic reaction chart for dissimilar metals. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); This occurs because aluminum is more. Stainless Steel And Aluminum Reaction.
From galvanizeit.org
Dissimilar Metal Corrosion with… American Galvanizers Association Stainless Steel And Aluminum Reaction Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid. Stainless steel (active) + aluminum. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Stainless steel and. Stainless Steel And Aluminum Reaction.
From www.sliderbase.com
Metals in industry Presentation Chemistry Stainless Steel And Aluminum Reaction Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid. If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. Learn why aluminum and stainless steel can corrode when coupled and how to avoid it. 10 easy steps on how. Stainless Steel And Aluminum Reaction.
From slidetodoc.com
CORROSION Principle of Corrosion Different types of Corrosion Stainless Steel And Aluminum Reaction Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. Find out the methods and materials to prevent galvanic. Stainless steel acts as the cathode, and aluminum acts as an anode. Stainless steel (active) + aluminum. Learn why aluminum and stainless. Stainless Steel And Aluminum Reaction.
From www.simpletwig.com
Galvanic Action Corrosion Prevention Architect's Blog Stainless Steel And Aluminum Reaction Stainless steel acts as the cathode, and aluminum acts as an anode. Learn how stainless steel and aluminium can corrode each other when in electrical contact and bridged by a conductive liquid. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. This occurs because aluminum is more chemically reactive than stainless steel and. Stainless Steel And Aluminum Reaction.
From www.metrosteel.com.au
Aluminium vs Stainless Steel What Are The Main Differences Metro Steel Stainless Steel And Aluminum Reaction Find out the methods and materials to prevent galvanic. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); Stainless steel (active) + aluminum. This occurs because aluminum is more chemically reactive than stainless steel and can corrode when exposed to. Stainless Steel And Aluminum Reaction.
From www.iqsdirectory.com
Aluminized Steel What Is It? How Is it Made? Advantages Stainless Steel And Aluminum Reaction Stainless steel and aluminum are both widely used metals, but they can undergo galvanic corrosion when in contact. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Learn why aluminum and stainless steel can corrode when coupled and how. Stainless Steel And Aluminum Reaction.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts Stainless Steel And Aluminum Reaction Stainless steel (active) + aluminum. There can be several reasons behind. If the surface area of aluminum is larger than stainless steel, corrosion of the aluminum surface is likely to occur. Stainless steel and aluminum can be used together but in most cases, both metals catch corrosion. Learn why aluminum and stainless steel can corrode when coupled and how to. Stainless Steel And Aluminum Reaction.
From pediaa.com
Difference Between Aluminum and Stainless Steel Properties, Use Stainless Steel And Aluminum Reaction 10 easy steps on how to prevent galvanic corrosion between aluminum and stainless steel step 1: Stainless steel (active) + aluminum. Below is a galvanic reaction chart for dissimilar metals. Aluminum negatively reacts to stainless steel but with a conducive environment (marine environment); Stainless steel acts as the cathode, and aluminum acts as an anode. This chart is designed to. Stainless Steel And Aluminum Reaction.