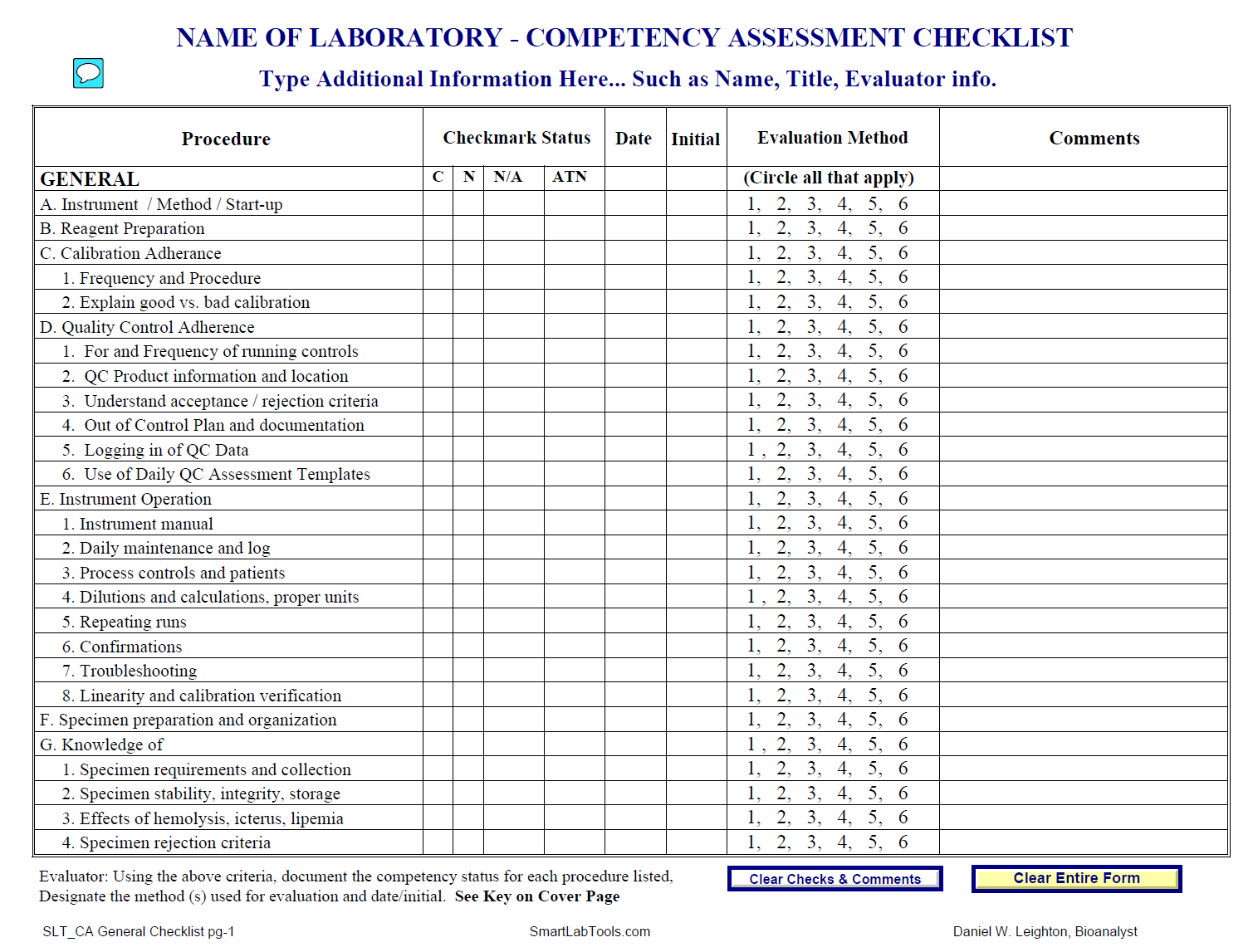
Cap Requirements For Method Validation . Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Other techniques include (1) assay of the current calibration materials as unknown. Our unique, reciprocal, peer inspection model benefits both the. Cap inspections are educational, not punitive. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. The following is a complete list of the cap accreditation checklists: Validation or verification process, it must be followed. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap accredited laboratories should comply with the current edition of the lap checklist requirements.
from exotijeyh.blob.core.windows.net
The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. Validation or verification process, it must be followed. Other techniques include (1) assay of the current calibration materials as unknown. The following is a complete list of the cap accreditation checklists: Cap inspections are educational, not punitive. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Our unique, reciprocal, peer inspection model benefits both the. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in.
Cap Requirements For Competency Assessment at Sharon Mart blog
Cap Requirements For Method Validation Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The following is a complete list of the cap accreditation checklists: Other techniques include (1) assay of the current calibration materials as unknown. Validation or verification process, it must be followed. Our unique, reciprocal, peer inspection model benefits both the. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap inspections are educational, not punitive. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to.
From www.slideserve.com
PPT CAP PowerPoint Presentation, free download ID6252598 Cap Requirements For Method Validation Our unique, reciprocal, peer inspection model benefits both the. The following is a complete list of the cap accreditation checklists: Validation or verification process, it must be followed. Cap inspections are educational, not punitive. Other techniques include (1) assay of the current calibration materials as unknown. Cap accredited laboratories should comply with the current edition of the lap checklist requirements.. Cap Requirements For Method Validation.
From mungfali.com
Validation Report Format Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap inspections are educational, not punitive. Other techniques include (1) assay of the current calibration materials as unknown. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Our unique, reciprocal, peer inspection model. Cap Requirements For Method Validation.
From www.slideshare.net
Cap guidelines 2010 Cap Requirements For Method Validation Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. The following is a complete list of the cap accreditation checklists: Cap inspections are educational, not punitive. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The cap accreditation checklists contain requirements for test method. Cap Requirements For Method Validation.
From www.slideshare.net
CAP 2010 Guidelines Cap Requirements For Method Validation Validation or verification process, it must be followed. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap inspections are educational, not punitive. The cap accreditation checklists contain requirements for test method validation and adverse event reporting,. Cap Requirements For Method Validation.
From www.slideshare.net
Significance of CAP accreditation Cap Requirements For Method Validation Other techniques include (1) assay of the current calibration materials as unknown. Cap inspections are educational, not punitive. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap accredited laboratories should comply with. Cap Requirements For Method Validation.
From exotijeyh.blob.core.windows.net
Cap Requirements For Competency Assessment at Sharon Mart blog Cap Requirements For Method Validation Other techniques include (1) assay of the current calibration materials as unknown. Cap inspections are educational, not punitive. The following is a complete list of the cap accreditation checklists: Validation or verification process, it must be followed. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. The cap accreditation. Cap Requirements For Method Validation.
From dtsreports.web.fc2.com
Cap Guidelines For Assay Validation Report Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Other techniques include (1) assay of the current calibration materials as unknown. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The following is a complete list of the cap accreditation checklists: Cap inspections are educational, not. Cap Requirements For Method Validation.
From www.slideserve.com
PPT Rehabilitation Services Administration PowerPoint Presentation Cap Requirements For Method Validation The following is a complete list of the cap accreditation checklists: Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system. Cap Requirements For Method Validation.
From studylib.net
15 Administrative Cap Requirements Cap Requirements For Method Validation The following is a complete list of the cap accreditation checklists: Other techniques include (1) assay of the current calibration materials as unknown. Our unique, reciprocal, peer inspection model benefits both the. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap accredited laboratories should comply with the current. Cap Requirements For Method Validation.
From www.researchgate.net
Summary of the major changes in the ASCO/CAP HER2 testing guidelines Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. The following is a complete list of the cap accreditation checklists: Cap inspections are educational, not punitive. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The cap accreditation checklists contain requirements for test method validation and. Cap Requirements For Method Validation.
From www.researchgate.net
2018 ASCO/CAP guidelines for HER2 dualprobe ISH clinical subgroups Cap Requirements For Method Validation Validation or verification process, it must be followed. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap inspections are educational, not punitive. Cap accredited laboratories should comply with the current. Cap Requirements For Method Validation.
From www.pdffiller.com
validation summary report example Doc Template pdfFiller Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The following is a complete list of the cap accreditation checklists: Cap inspections are educational, not punitive. Validation or verification process, it must be followed. The cap accreditation. Cap Requirements For Method Validation.
From www.youtube.com
CapScore™ Test Home Collection Instructions YouTube Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap inspections are educational, not punitive. Validation or verification process, it must be followed. Cap accredited laboratories should comply with the current. Cap Requirements For Method Validation.
From www.slideserve.com
PPT Understanding Nonprofits and the National Forest Foundation (NFF Cap Requirements For Method Validation Other techniques include (1) assay of the current calibration materials as unknown. Validation or verification process, it must be followed. The following is a complete list of the cap accreditation checklists: Cap accredited laboratories should comply with the current edition of the lap checklist requirements. Cap inspections are educational, not punitive. Our unique, reciprocal, peer inspection model benefits both the.. Cap Requirements For Method Validation.
From www.presentationeze.com
FDA GMP QSR Equipment and Maintenance PresentationEZE Cap Requirements For Method Validation Our unique, reciprocal, peer inspection model benefits both the. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap inspections are educational, not punitive. The following is a complete list of the cap accreditation checklists: Other techniques include (1) assay of the current calibration materials as unknown. The cap accreditation checklists. Cap Requirements For Method Validation.
From www.scribd.com
Corrective Action Plan (CAP) Implementation Evaluation Checklist Cap Requirements For Method Validation Other techniques include (1) assay of the current calibration materials as unknown. Our unique, reciprocal, peer inspection model benefits both the. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap inspections are. Cap Requirements For Method Validation.
From www.slideserve.com
PPT E pluribus unum Regulatory Perspectives on Validating Moderately Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Validation or verification process, it must be followed. Cap accredited laboratories should comply with the current edition of the lap checklist requirements.. Cap Requirements For Method Validation.
From exoocgzyu.blob.core.windows.net
Cap Sop Requirements at Jason Oldham blog Cap Requirements For Method Validation Cap inspections are educational, not punitive. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Our unique, reciprocal, peer inspection model benefits both the. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. The following is a complete list of. Cap Requirements For Method Validation.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. Cap inspections are educational, not punitive. Our unique, reciprocal, peer inspection model benefits both the. The cap accreditation checklists contain requirements for test method validation and adverse event. Cap Requirements For Method Validation.
From www.scribd.com
CAP Accreditation Requirements For Validation of Laboratory Tests PDF Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Validation or verification process, it must be followed. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The following. Cap Requirements For Method Validation.
From www.researchgate.net
Schematic workflow of the validation study. Download Scientific Diagram Cap Requirements For Method Validation Cap inspections are educational, not punitive. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. The following is a complete list of the cap accreditation checklists: Other techniques include (1) assay of the current calibration materials as unknown. Cap accredited laboratories should comply with the current edition of the lap checklist. Cap Requirements For Method Validation.
From www.slideserve.com
PPT E pluribus unum Regulatory Perspectives on Validating Moderately Cap Requirements For Method Validation Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The following is a complete list of the cap accreditation checklists: Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Our unique, reciprocal, peer inspection model benefits both the. Other techniques include (1) assay of. Cap Requirements For Method Validation.
From www.slideserve.com
PPT Method Validation Studies It’s a Jungle out There! PowerPoint Cap Requirements For Method Validation Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap inspections are educational, not punitive. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The cap. Cap Requirements For Method Validation.
From newsroom.cap.org
CAP Updates Guidelines for Validation of Immunohistochemical Assays Cap Requirements For Method Validation Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Other techniques include (1) assay of the current calibration materials as unknown. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The cap accreditation checklists contain requirements for test method validation and adverse event reporting,. Cap Requirements For Method Validation.
From www.americanpharmaceuticalreview.com
Analytical Method Validation for Quality Assurance and Process Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Other techniques include (1) assay of the current calibration materials as unknown. Our unique, reciprocal, peer inspection model benefits both the. Cap. Cap Requirements For Method Validation.
From www.youtube.com
CapCut System Requirements Cap Cut Requirements Minimum Cap Requirements For Method Validation Our unique, reciprocal, peer inspection model benefits both the. Other techniques include (1) assay of the current calibration materials as unknown. Validation or verification process, it must be followed. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. Cap inspections are educational, not punitive. The cap accreditation checklists contain requirements for test method validation and. Cap Requirements For Method Validation.
From www.researchgate.net
Respondents by Country and Availability of National Guidelines for CaP Cap Requirements For Method Validation Validation or verification process, it must be followed. Our unique, reciprocal, peer inspection model benefits both the. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap accredited laboratories should comply with the. Cap Requirements For Method Validation.
From www.sampletemplates.com
10+ Validation Plan Templates Sample Templates Cap Requirements For Method Validation Other techniques include (1) assay of the current calibration materials as unknown. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap accredited laboratories should comply with the current edition of the lap. Cap Requirements For Method Validation.
From www.slideserve.com
PPT New Guidelines for CAP Diagnosis and Therapy A View from North Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Other techniques include (1) assay of the current calibration materials as unknown. The following is a complete list of the cap accreditation checklists: The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Cap. Cap Requirements For Method Validation.
From www.researchgate.net
ISO 22870 Management requirements with cross reference to CPA standards Cap Requirements For Method Validation Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Validation or verification process, it must be followed. Cap accredited laboratories should comply with the current edition of the lap checklist requirements. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to.. Cap Requirements For Method Validation.
From www.scribd.com
Cap Accreditation Checklists PDF Pathology Serology Cap Requirements For Method Validation Cap inspections are educational, not punitive. Other techniques include (1) assay of the current calibration materials as unknown. Our unique, reciprocal, peer inspection model benefits both the. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. The following is a complete list of the cap accreditation checklists: Cap accredited. Cap Requirements For Method Validation.
From www.sampletemplate.my.id
Validation Certificate Template Sampletemplate.my.id Cap Requirements For Method Validation Our unique, reciprocal, peer inspection model benefits both the. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Other techniques include (1) assay of the current calibration materials as unknown. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Validation. Cap Requirements For Method Validation.
From www.researchgate.net
Global CAP requirements and pledges (2005) across main sectors Cap Requirements For Method Validation Cap accredited laboratories should comply with the current edition of the lap checklist requirements. Other techniques include (1) assay of the current calibration materials as unknown. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. Cap Requirements For Method Validation.
From www.smartlabtools.com
SmartLabTools Method Validation Cap Requirements For Method Validation The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap inspections are educational, not punitive. The following is a complete list of the cap accreditation checklists: Our unique, reciprocal, peer inspection. Cap Requirements For Method Validation.
From www.slideserve.com
PPT Method Validation Studies It’s a Jungle out There! PowerPoint Cap Requirements For Method Validation Other techniques include (1) assay of the current calibration materials as unknown. The cap accreditation checklists contain requirements for test method validation and adverse event reporting, which are used to. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Our unique, reciprocal, peer inspection model benefits both the. Validation. Cap Requirements For Method Validation.