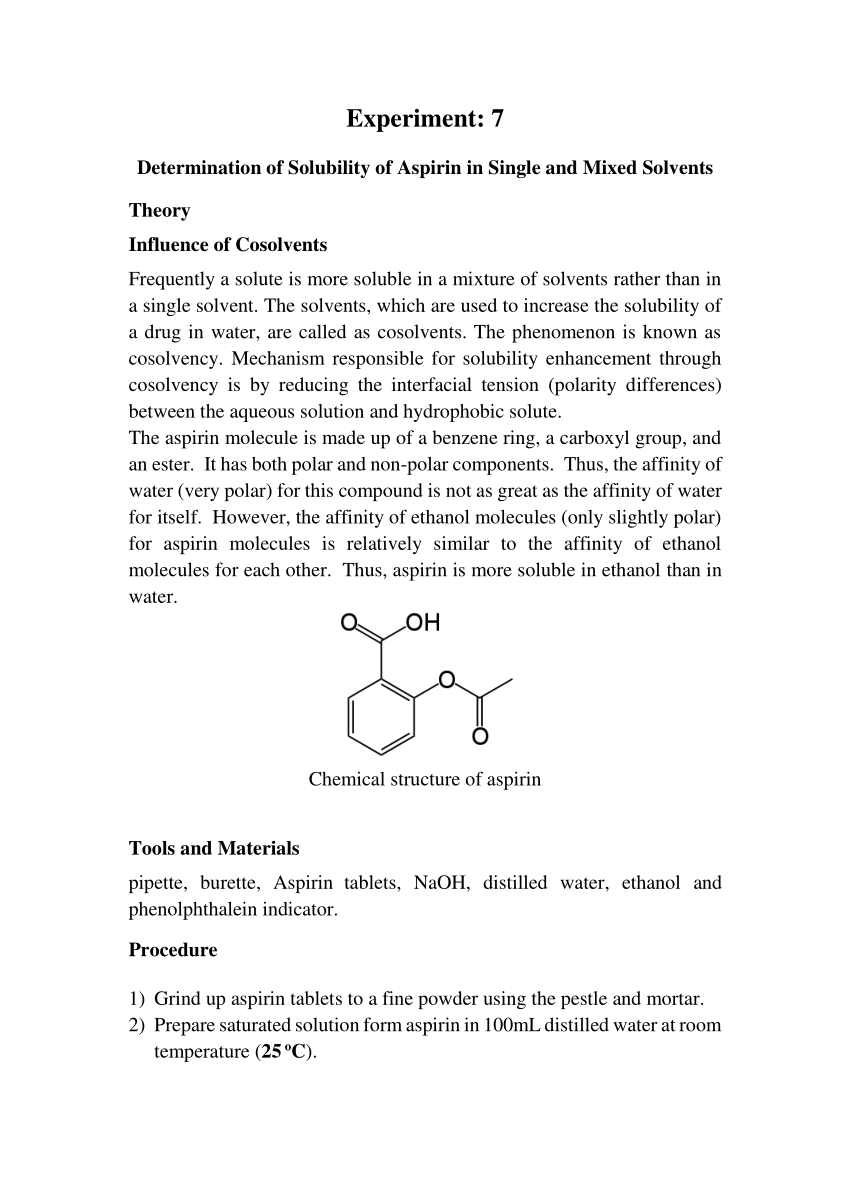
Aspirin + Water Equation . Aspirin is slightly soluble in water: Is aspirin an acid or base? Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4.
from www.researchgate.net
The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Aspirin is slightly soluble in water: Is aspirin an acid or base? The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group.
(PDF) Experiment 7 Determination of Solubility of Aspirin in Single and Mixed Solvents
Aspirin + Water Equation The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin is slightly soluble in water: The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Is aspirin an acid or base?
From www.alamy.it
Struttura chimica dell'aspirina, Anatomia dell'aspirina, struttura molecolare dell'aspirina Aspirin + Water Equation Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Is aspirin an acid or base? Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Aspirin + Water Equation.
From www.chegg.com
Solved water? 5. Aspirin gradually reacts with moisture in Aspirin + Water Equation Aspirin is slightly soluble in water: Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The solubility of aspirin. Aspirin + Water Equation.
From www.dreamstime.com
Drawn Molecule and Formula of Aspirin Stock Vector Illustration of icon, formula 240444646 Aspirin + Water Equation Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is slightly soluble in water: The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional. Aspirin + Water Equation.
From www.tessshebaylo.com
Chemical Equation Representing Synthesis Of Aspirin From Acetyl Chloride Tessshebaylo Aspirin + Water Equation Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Aspirin is slightly soluble in water: Is aspirin an acid or base? The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8. Aspirin + Water Equation.
From studylib.net
Experiment AcidBase Titration of Aspirin Aspirin + Water Equation Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Aspirin is slightly soluble in water: Is aspirin an acid or base? The solubility of aspirin in water is 0.33 grams. Aspirin + Water Equation.
From drugsdetails.com
Aspirin Drugs Details Aspirin + Water Equation Aspirin is slightly soluble in water: Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Is aspirin an acid or base? Acetylsalicylic acid. Aspirin + Water Equation.
From www.dreamstime.com
Handwriting Chemical Formula of Aspirin Stock Image Image of studying, chemical 150037847 Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Aspirin is slightly soluble in water: Is aspirin an acid or base? The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Acetylsalicylic acid. Aspirin + Water Equation.
From www.shutterstock.com
Aspirin Structural Formula Acetylsalicylic Acid C9h8o4 vector de stock (libre de regalías Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Is aspirin an acid or base? Aspirin is slightly soluble in water: Aspirin. Aspirin + Water Equation.
From www.coursehero.com
[Solved] What is the chemical structure of aspirin? And what functional... Course Hero Aspirin + Water Equation Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Is aspirin an acid or base? The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Acetylsalicylic acid commonly known as aspirin is a. Aspirin + Water Equation.
From kr.freepik.com
아스피린 화학 화학식 구조 벡터 프리미엄 벡터 Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is slightly soluble in water: Is aspirin an acid or base? Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester. Aspirin + Water Equation.
From it.dreamstime.com
Struttura dell'aspirina illustrazione vettoriale. Illustrazione di formula 26227597 Aspirin + Water Equation Is aspirin an acid or base? Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is slightly soluble in water: The mechanism of action of aspirin is as a. Aspirin + Water Equation.
From www.youtube.com
Solubility of Aspirin (Explained) YouTube Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Is aspirin an acid or base? Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Aspirin is slightly soluble in water: Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8. Aspirin + Water Equation.
From es.vecteezy.com
Molécula de fármaco aspirina de ácido acetilsalicílico. fórmula esquelética sobre fondo blanco Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is slightly soluble in water: The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Aspirin (acetylsalicylic acid) is an aromatic compound. Aspirin + Water Equation.
From www.chegg.com
Solved Give the reaction mechanism for the synthesis of Aspirin + Water Equation Is aspirin an acid or base? Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional. Aspirin + Water Equation.
From www.vecteezy.com
Vector Skeletal formula of Aspirin. Drug chemical molecule. 6589544 Vector Art at Vecteezy Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Aspirin is slightly soluble in water: Is aspirin an acid or base? The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Acetylsalicylic acid. Aspirin + Water Equation.
From www.chegg.com
Solved 6. In the synthesis of aspirin we react salicylic Aspirin + Water Equation Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Is aspirin an acid or base? Acetylsalicylic acid commonly known as aspirin is a. Aspirin + Water Equation.
From www.researchgate.net
Paracetamol and aspirin pathway. Side reactions solvation of aspirin... Download Scientific Aspirin + Water Equation Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin is slightly soluble in water: Is aspirin an acid or base? Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester. Aspirin + Water Equation.
From www.dreamstime.com
Molecular Structure Of Aspirin Stock Illustration Illustration of atom, chemistry 13137869 Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Is aspirin an acid or base? Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Aspirin is slightly soluble in water: Aspirin. Aspirin + Water Equation.
From solvedlib.com
7. What is the pKa of aspirin? Of water?8. Based on y… SolvedLib Aspirin + Water Equation Is aspirin an acid or base? Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is slightly soluble in water: Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. The mechanism of action of aspirin is as a. Aspirin + Water Equation.
From www.researchgate.net
(PDF) Experiment 7 Determination of Solubility of Aspirin in Single and Mixed Solvents Aspirin + Water Equation Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Aspirin is slightly soluble in water: The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Is aspirin an acid or base? The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Acetylsalicylic acid. Aspirin + Water Equation.
From es.dreamstime.com
Estructura de aspirina stock de ilustración. Ilustración de carbono 213070912 Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Is aspirin an acid or base? The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Aspirin (acetylsalicylic acid) is an aromatic compound. Aspirin + Water Equation.
From www.studocu.com
Lab 4 Synthesis of Aspirin Lab Partner_____________________________ Synthesis of Aspirin Week Aspirin + Water Equation The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Aspirin is slightly soluble in water: Is aspirin an acid or base? The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Acetylsalicylic acid. Aspirin + Water Equation.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID6186176 Aspirin + Water Equation Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin is slightly soluble in water: Is aspirin an acid or base? Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester. Aspirin + Water Equation.
From www.coursehero.com
Solved What is the equation for the reaction that can occur with aspirin in water? Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin is slightly soluble in water: Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Is aspirin an acid or base? The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Acetylsalicylic acid. Aspirin + Water Equation.
From www.shutterstock.com
444 imágenes de Aspirin formula Imágenes, fotos y vectores de stock Shutterstock Aspirin + Water Equation Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is slightly soluble in water: Is aspirin an acid or base? The mechanism of action of aspirin is as a. Aspirin + Water Equation.
From www.numerade.com
SOLVED Write balanced reaction equations for the reactions involved (a) when aspirin dissolves Aspirin + Water Equation Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Is aspirin an acid or base? The solubility of aspirin. Aspirin + Water Equation.
From www.dreamstime.com
Aspirin Stock Vector Image 42307258 Aspirin + Water Equation Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Is aspirin an acid or base? The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Aspirin + Water Equation.
From study.com
Hydrolysis of Aspirin Overview, Reactions & Mechanism Lesson Aspirin + Water Equation Is aspirin an acid or base? Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Aspirin + Water Equation.
From www.tessshebaylo.com
Chemical Equation Synthesis Of Aspirin From Acetyl Chloride Tessshebaylo Aspirin + Water Equation Is aspirin an acid or base? Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The solubility of aspirin in water is 0.33 grams per 100 ml water at room. Aspirin + Water Equation.
From ar.inspiredpencil.com
Aspirin Lewis Structure Aspirin + Water Equation The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Is aspirin an acid or base? Acetylsalicylic acid commonly known as aspirin is a. Aspirin + Water Equation.
From www.numerade.com
SOLVED The distribution coefficient; Kpx between ether and water for aspirin (acetylsalicylic Aspirin + Water Equation Aspirin is slightly soluble in water: Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Is aspirin an acid or base? Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. The mechanism of action of aspirin is as a. Aspirin + Water Equation.
From www.coursehero.com
[Solved] The distribution coefficient of aspirin in ether/water at 25°C is... Course Hero Aspirin + Water Equation Aspirin is slightly soluble in water: The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Is aspirin an acid or base? Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8. Aspirin + Water Equation.
From www.alamyimages.fr
Formule topologique l'aspirine Banque d'images noir et blanc Alamy Aspirin + Water Equation The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Is aspirin an acid or base? Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is slightly soluble in water: The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin. Aspirin + Water Equation.
From www.chegg.com
Solved 2. Learning how fast the hydrolysis of aspirin occurs Aspirin + Water Equation Aspirin is slightly soluble in water: Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Is aspirin an acid or base? The mechanism of action of aspirin is as a. Aspirin + Water Equation.
From mugeek.vidalondon.net
Chemical Makeup Of Aspirin Mugeek Vidalondon Aspirin + Water Equation Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. Aspirin is slightly soluble in water: Aspirin (acetylsalicylic acid) is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. The solubility of aspirin. Aspirin + Water Equation.