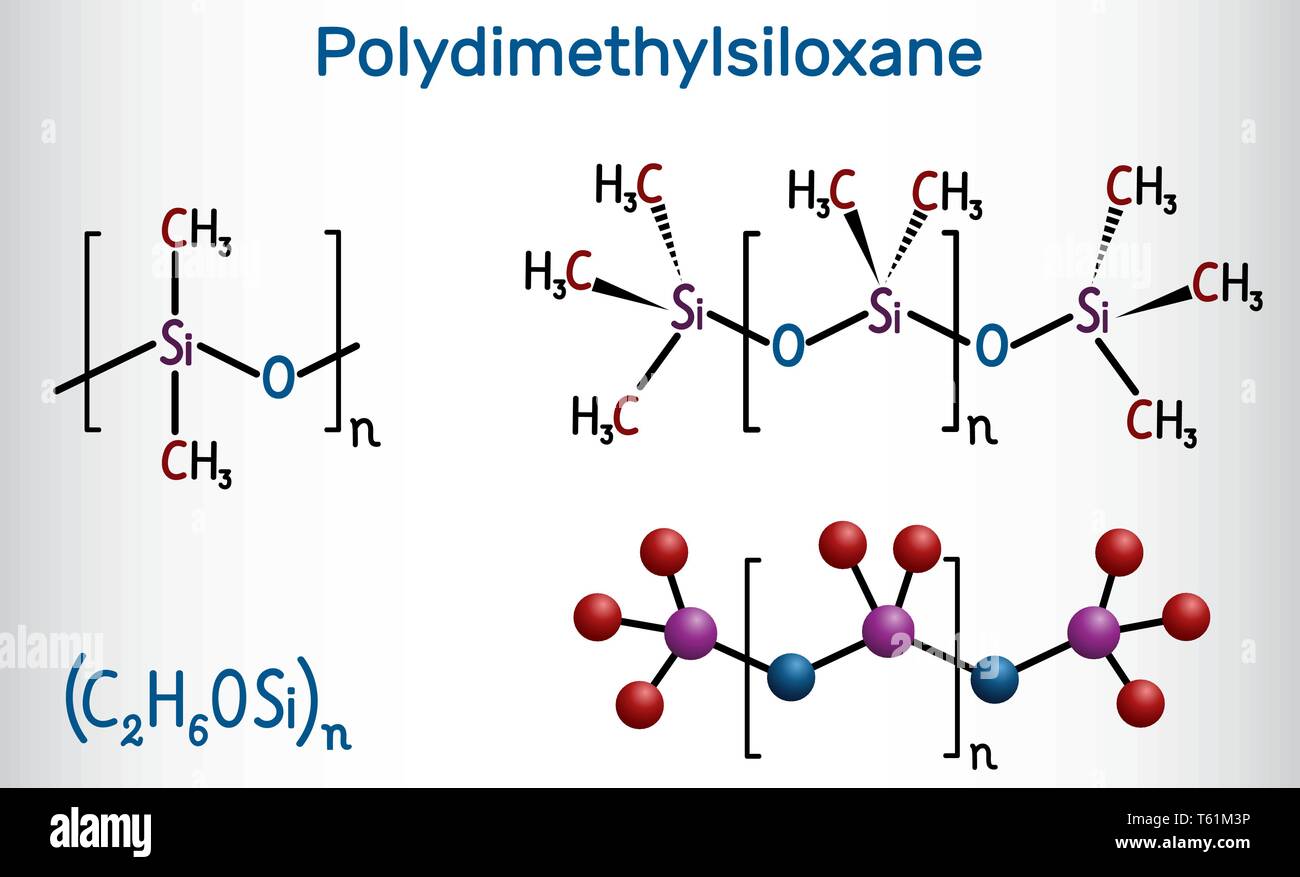
Silicone Rubber Molecular Structure . High binding energy, intermolecular force and coil formation. Silicone rubbers are widely used in the medical. Solid silicone rubber or high. They are available in three main forms: In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Its molecular structure is characterized by the following: The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers.
from ar.inspiredpencil.com
They are available in three main forms: A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Its molecular structure is characterized by the following: Silicone rubbers are widely used in the medical. High binding energy, intermolecular force and coil formation. Solid silicone rubber or high.
Silicone Structure
Silicone Rubber Molecular Structure The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Silicone rubbers are widely used in the medical. The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Solid silicone rubber or high. Its molecular structure is characterized by the following: They are available in three main forms: High binding energy, intermolecular force and coil formation.
From www.researchgate.net
The schematic composition and structure of the silicone resin with... Download Scientific Diagram Silicone Rubber Molecular Structure Silicone rubbers are widely used in the medical. Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Solid silicone rubber or high. Its molecular structure is characterized by. Silicone Rubber Molecular Structure.
From www.mdpi.com
Polymers Free FullText Properties of Silicone RubberBased Composites Reinforced with Few Silicone Rubber Molecular Structure In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. They are available in three main forms: Silicone rubbers are widely used in the medical. Solid silicone rubber or. Silicone Rubber Molecular Structure.
From www.elementalchemistry.in
ELEMENTAL CHEMISTRY Vulcanization of Rubber Silicone Rubber Molecular Structure Its molecular structure is characterized by the following: Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. They are available in three main forms: The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. High binding energy, intermolecular force and coil formation. In organosilicon and polymer chemistry, a silicone. Silicone Rubber Molecular Structure.
From polymer360.blogspot.com
Silicone rubbers Silicone Rubber Molecular Structure Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Solid silicone rubber or high. Silicone rubbers are widely used in the medical. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Its molecular structure is characterized by the following: In organosilicon and polymer chemistry,. Silicone Rubber Molecular Structure.
From lookfordiagnosis.com
Silicone Elastomers; Elastomers, Silicone; Rubber Silicone; Silicone Rubber Silicone Rubber Molecular Structure Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Solid silicone rubber or high. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Silicone rubbers are widely used in the medical. High binding energy, intermolecular force and. Silicone Rubber Molecular Structure.
From www.inmr.com
Chemistry & Properties of Silicones Silicone Rubber Molecular Structure The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Solid silicone rubber or high. High binding energy, intermolecular force and coil formation. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of. Silicone Rubber Molecular Structure.
From www.mdpi.com
Molecules Free FullText Electric Field Intensity Effects on the Microstructural Silicone Rubber Molecular Structure They are available in three main forms: Solid silicone rubber or high. Its molecular structure is characterized by the following: High binding energy, intermolecular force and coil formation. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Natural rubber is the starting material for introduction. Silicone Rubber Molecular Structure.
From siliconecloth.com
Liquid Silicone Rubber Silicone Cloth Silicone Rubber Molecular Structure The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Solid silicone rubber or high. High binding energy, intermolecular force and coil formation. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. They are available in three main forms: Its molecular structure is characterized by the following: Natural rubber. Silicone Rubber Molecular Structure.
From chem.libretexts.org
Silicon and Group 14 Elements Chemistry LibreTexts Silicone Rubber Molecular Structure The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Solid silicone rubber or high. Its molecular structure is characterized by the following: They are available in three main forms: In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Silicone rubbers are. Silicone Rubber Molecular Structure.
From www.slideserve.com
PPT Alloplastik Metaryaller PowerPoint Presentation ID1183168 Silicone Rubber Molecular Structure Its molecular structure is characterized by the following: A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Solid silicone rubber or high. They are available in three main forms: Silicone rubbers are widely used in the medical. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating. Silicone Rubber Molecular Structure.
From www.hmdsilicone.com
News What is silicone?Shenzhen Huimingde Silicone Rubber Products Co., Ltd. Silicone Rubber Molecular Structure Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Its molecular structure is characterized by the following: High binding energy, intermolecular force and coil formation. They are available in three main forms: Solid silicone rubber or high. The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Silicone rubbers. Silicone Rubber Molecular Structure.
From www.peoi.org
Chapter 16 Section F Polymers Silicone Rubber Molecular Structure The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Its molecular structure is characterized by the following: In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r =. Silicone Rubber Molecular Structure.
From www.dakenchem.com
Dakenchem에서 전도성 실리콘 고무 실리콘 신청 Silicone Rubber Molecular Structure The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. They are available in three main forms: A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Solid silicone rubber or high. Silicone rubbers are widely used in the medical. High binding energy, intermolecular force and coil formation. Its molecular. Silicone Rubber Molecular Structure.
From www.key-plast.com
Identifying silicone and rubber Taizhou Huangyan Fow Mould Co., Ltd. Silicone Rubber Molecular Structure Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). The molecular. Silicone Rubber Molecular Structure.
From www.mdpi.com
Polymers Free FullText Influence of Curing Agent Amount on Properties of Dynamic Vulcanized Silicone Rubber Molecular Structure Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Its molecular structure is characterized by the following: A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. They are available in three. Silicone Rubber Molecular Structure.
From www.istockphoto.com
A Design Of A Silicone Molecule Stock Photo Download Image Now Silicone, Silicon, Molecule Silicone Rubber Molecular Structure Silicone rubbers are widely used in the medical. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Solid silicone rubber or high. A siloxane backbone (a chain of silicon and oxygen) an. Silicone Rubber Molecular Structure.
From www.iqsdirectory.com
Silicone Rubber Molding Types, Materials, Processes & Uses Silicone Rubber Molecular Structure High binding energy, intermolecular force and coil formation. They are available in three main forms: Its molecular structure is characterized by the following: Solid silicone rubber or high. The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. In organosilicon. Silicone Rubber Molecular Structure.
From classnotes.org.in
Silicones Chemistry, Class 11, pBlock Elements Silicone Rubber Molecular Structure Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Silicone rubbers are widely used in the medical. High binding energy, intermolecular force and coil formation. The molecular structure,. Silicone Rubber Molecular Structure.
From cpb.iphy.ac.cn
Molecular dynamics simulation of thermal conductivity of silicone rubber Silicone Rubber Molecular Structure High binding energy, intermolecular force and coil formation. They are available in three main forms: Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Silicone rubbers are widely used in the medical. Its molecular structure. Silicone Rubber Molecular Structure.
From www.aokthermal.com
Thermal Interface Materials Experts and SupplierSilicone Rubber Thermal Pads Silicone Rubber Molecular Structure A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Solid silicone rubber or high. The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. They are available in three main forms: High binding energy, intermolecular force and coil formation. Silicone rubbers are widely used in the medical. In organosilicon. Silicone Rubber Molecular Structure.
From www.researchgate.net
Optimized molecular model of silicone rubber Download Scientific Diagram Silicone Rubber Molecular Structure Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Solid silicone rubber or high. They are available in three main forms: The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane. Silicone Rubber Molecular Structure.
From www.researchgate.net
Basic silicone rubber structure. 41 Download Scientific Diagram Silicone Rubber Molecular Structure Silicone rubbers are widely used in the medical. They are available in three main forms: In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Solid silicone rubber or high. Its molecular structure is characterized by the following: A siloxane backbone (a chain of silicon and. Silicone Rubber Molecular Structure.
From tbbonding.com
Don't glue Silicone rubber, BOND Silicone rubber TechBond Solutions Silicone Rubber Molecular Structure In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Silicone rubbers are widely used in the medical. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Natural rubber is the starting material for introduction of chemistries that. Silicone Rubber Molecular Structure.
From www.researchgate.net
Molecular chain structure of (a) silicone oil. (b) Methyl‐vinyl... Download Scientific Diagram Silicone Rubber Molecular Structure High binding energy, intermolecular force and coil formation. Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. They are available in three main forms: Solid silicone rubber or high. In organosilicon and polymer chemistry, a. Silicone Rubber Molecular Structure.
From ar.inspiredpencil.com
Silicon Molecular Structure Silicone Rubber Molecular Structure High binding energy, intermolecular force and coil formation. They are available in three main forms: A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Solid silicone rubber or high. The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Natural rubber is the starting material for introduction of chemistries. Silicone Rubber Molecular Structure.
From www.researchgate.net
Route for the synthesis of RTV silicone rubber. (i) Synthetic route for... Download Scientific Silicone Rubber Molecular Structure Its molecular structure is characterized by the following: Solid silicone rubber or high. High binding energy, intermolecular force and coil formation. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r =. Silicone Rubber Molecular Structure.
From www.mdpi.com
Polymers Free FullText Synthesis and Characterization of Room Temperature Vulcanized Silicone Rubber Molecular Structure High binding energy, intermolecular force and coil formation. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Silicone rubbers are widely used in the medical. Solid silicone rubber. Silicone Rubber Molecular Structure.
From ar.inspiredpencil.com
Silicone Structure Silicone Rubber Molecular Structure Solid silicone rubber or high. Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Its molecular structure is characterized by the following: In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed. Silicone Rubber Molecular Structure.
From mungfali.com
Crystal Structure Of Silicon Silicone Rubber Molecular Structure Silicone rubbers are widely used in the medical. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Solid silicone rubber or high. A siloxane backbone (a chain of silicon and oxygen) an. Silicone Rubber Molecular Structure.
From www.researchgate.net
Scheme of formation of silicone rubber matrix with durably builtin... Download Scientific Diagram Silicone Rubber Molecular Structure In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Silicone rubbers are widely used in. Silicone Rubber Molecular Structure.
From www.avkvalves.eu
Insight into the complexity of rubber formulation AVK International Silicone Rubber Molecular Structure High binding energy, intermolecular force and coil formation. The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Silicone rubbers are widely used in the medical. In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of. Silicone Rubber Molecular Structure.
From www.researchgate.net
The structure of Dimethyl polysiloxane “Silicon Rubber”. Download Scientific Diagram Silicone Rubber Molecular Structure High binding energy, intermolecular force and coil formation. They are available in three main forms: In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Its molecular structure is characterized by the following:. Silicone Rubber Molecular Structure.
From mavink.com
Pdms Structure Silicone Rubber Molecular Structure Solid silicone rubber or high. High binding energy, intermolecular force and coil formation. Its molecular structure is characterized by the following: A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. Silicone rubbers are widely used in the medical. In. Silicone Rubber Molecular Structure.
From inpart24.com
22 Different Types of Rubber Knowledge Base Inpart Silicone Rubber Molecular Structure In organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (−o−r2si−o−sir2−, where r = organic group). Natural rubber is the starting material for introduction of chemistries that introduce damping, abrasion resistance and higher. Its molecular structure is characterized by the following: They are available in three main forms: Solid silicone rubber or. Silicone Rubber Molecular Structure.
From ar.inspiredpencil.com
Silicone Structure Silicone Rubber Molecular Structure Silicone rubbers are widely used in the medical. They are available in three main forms: High binding energy, intermolecular force and coil formation. The molecular structure, viscosity, and processing methods are factors for classifying silicone rubbers. A siloxane backbone (a chain of silicon and oxygen) an organic component is attached to the silicon. Its molecular structure is characterized by the. Silicone Rubber Molecular Structure.