Specific Heat Capacity Test Question . Specific heat capacity quiz a. Test yourself on this topic. What is the specific heat capacity of water? What is the name of the variable that tells you how much heat energy is required to change the. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the. The specific heat capacity is the amount of heat energy required to raise the. What is the difference between thermal. This topic is designed as an interactive quiz. If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. Specific heat capacity test questions. What does it mean if water has a higher specific heat capacity than oil? The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×.
from zipworksheet.com
The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. Specific heat capacity test questions. Specific heat capacity quiz a. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the. Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. Test yourself on this topic. What is the specific heat capacity of water? The specific heat capacity is the amount of heat energy required to raise the. If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? What is the name of the variable that tells you how much heat energy is required to change the.
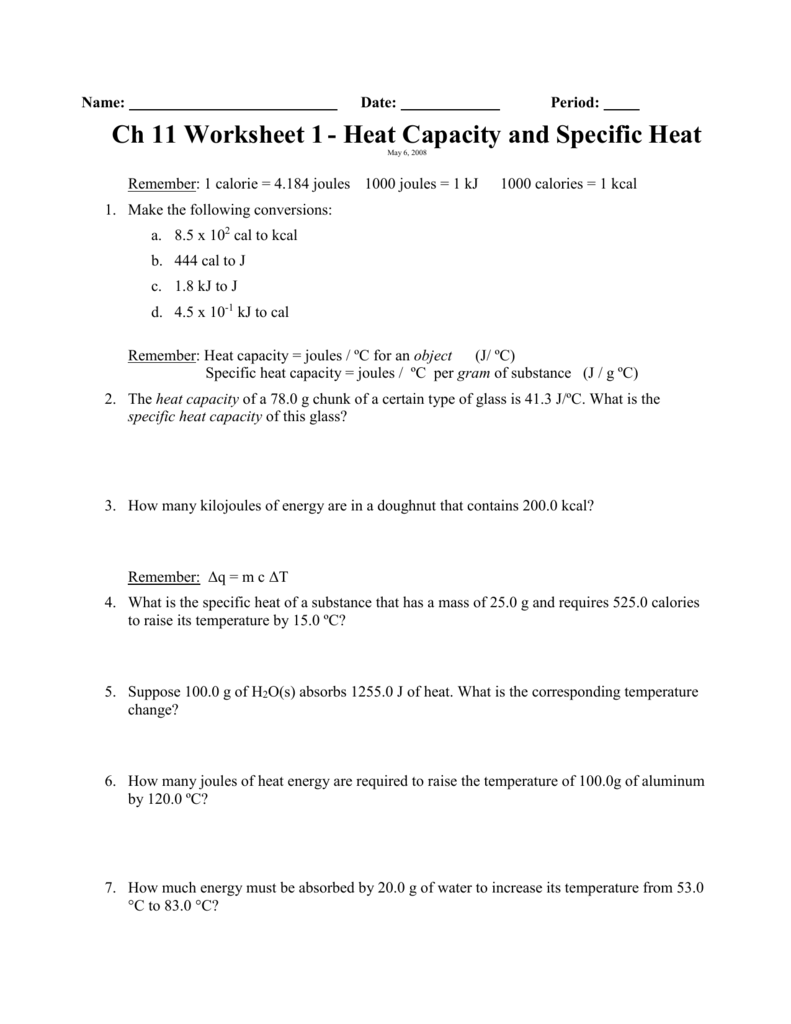
Specific Heat Worksheet Answers
Specific Heat Capacity Test Question The specific heat capacity is the amount of heat energy required to raise the. Specific heat capacity test questions. If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the. What is the difference between thermal. Test yourself on this topic. The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. Specific heat capacity quiz a. What is the name of the variable that tells you how much heat energy is required to change the. This topic is designed as an interactive quiz. What is the specific heat capacity of water? The specific heat capacity is the amount of heat energy required to raise the. What does it mean if water has a higher specific heat capacity than oil?
From www.studocu.com
Lab 2 Specific Heat Capacity 2 Physics 4C Spring 2023 Lab 2 Specific Heat Capacity Test Question Test yourself on this topic. Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. What is the specific heat capacity of water? Specific heat capacity test questions. What is the name of the variable that tells you how much heat energy is required to change the. The specific heat capacity is the amount of heat. Specific Heat Capacity Test Question.
From www.youtube.com
Specific Latent Heat and Heat Capacity Exam Question YouTube Specific Heat Capacity Test Question What is the specific heat capacity of water? What is the difference between thermal. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the. Specific heat capacity test questions. Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. The specific. Specific Heat Capacity Test Question.
From studylib.net
Lab 11b Specific Heat & Heat Capacity Specific Heat Capacity Test Question What is the difference between thermal. What does it mean if water has a higher specific heat capacity than oil? The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. This topic is designed as an interactive quiz. Specific heat capacity test questions. What is the name of the variable. Specific Heat Capacity Test Question.
From www.studypool.com
SOLUTION Physics notes heat capacity and specific heat capacity Specific Heat Capacity Test Question If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? This topic is designed as an interactive quiz. Test yourself on this topic. Specific heat capacity test questions. What is the difference between thermal. The thermal energy change (gain or loss) in a substance is equal to the (amount of. Specific Heat Capacity Test Question.
From www.hanlin.com
Edexcel IGCSE Physics 复习笔记 5.2.4 Specific Heat Capacity翰林国际教育 Specific Heat Capacity Test Question If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? Specific heat capacity test questions. What is the difference between thermal. What is the specific heat capacity of water? The specific heat capacity is the amount of heat energy required to raise the. This topic is designed as an interactive. Specific Heat Capacity Test Question.
From www.tes.com
Specific Heat Capacity Required Practical Exam Style Questions Specific Heat Capacity Test Question What is the difference between thermal. Test yourself on this topic. This topic is designed as an interactive quiz. What is the specific heat capacity of water? Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in. Specific Heat Capacity Test Question.
From studylib.net
questions involving specific and latent heat Specific Heat Capacity Test Question Specific heat capacity test questions. What does it mean if water has a higher specific heat capacity than oil? What is the name of the variable that tells you how much heat energy is required to change the. Specific heat capacity quiz a. What is the specific heat capacity of water? If something has a high specific heat capacity, why. Specific Heat Capacity Test Question.
From quizizz.com
50+ Measurement and Capacity worksheets for 7th Class on Quizizz Free Specific Heat Capacity Test Question C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the. What is the difference between thermal. If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? What is the name of the variable that tells. Specific Heat Capacity Test Question.
From studylib.net
1.3 Specific Heat Capacity Specific Heat Capacity Test Question What is the specific heat capacity of water? Specific heat capacity test questions. The specific heat capacity is the amount of heat energy required to raise the. This topic is designed as an interactive quiz. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the.. Specific Heat Capacity Test Question.
From askfilo.com
Capacity Heat capacitySpecific heat capacity1. It is the amount of heat e.. Specific Heat Capacity Test Question What does it mean if water has a higher specific heat capacity than oil? The specific heat capacity is the amount of heat energy required to raise the. Specific heat capacity quiz a. The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. If something has a high specific heat. Specific Heat Capacity Test Question.
From brainly.in
Specific heat capacity of the substance is decreasing with increase in Specific Heat Capacity Test Question Specific heat capacity test questions. If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? This topic is designed as an interactive quiz. What is the difference between thermal. The specific heat capacity is the amount of heat energy required to raise the. What does it mean if water has. Specific Heat Capacity Test Question.
From www.docsity.com
Heat Transfer Specific Heat Capacity and Specific Latent Heat Worksheet Specific Heat Capacity Test Question What is the specific heat capacity of water? Specific heat capacity test questions. This topic is designed as an interactive quiz. What is the name of the variable that tells you how much heat energy is required to change the. What does it mean if water has a higher specific heat capacity than oil? The specific heat capacity is the. Specific Heat Capacity Test Question.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. Specific Heat Capacity Test Question If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. What does it mean if water has a higher specific heat capacity than oil? This topic is designed as an interactive quiz. Specific heat capacity quiz. Specific Heat Capacity Test Question.
From docs.google.com
Specific Heat Capacity Questions 1 (with answers) Google Docs Specific Heat Capacity Test Question If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? What does it mean if water has a higher specific heat capacity than oil? Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. What is the difference between thermal. Specific heat capacity quiz a. This. Specific Heat Capacity Test Question.
From www.youtube.com
How to solve very hard specific heat capacity and latent heat numerical Specific Heat Capacity Test Question Specific heat capacity quiz a. Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. What is the difference between thermal. If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? What does it mean if water has a higher specific heat capacity than oil? The. Specific Heat Capacity Test Question.
From www.youtube.com
Specific heat capacity YouTube Specific Heat Capacity Test Question What does it mean if water has a higher specific heat capacity than oil? This topic is designed as an interactive quiz. The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. Specific heat capacity quiz a. What is the difference between thermal. Specific heat capacity test questions. Test yourself. Specific Heat Capacity Test Question.
From rootsphysics.blogspot.com
Past Papers Physics OLevels and IGCSE Specific heat capacity and Specific Heat Capacity Test Question The specific heat capacity is the amount of heat energy required to raise the. Specific heat capacity quiz a. What is the name of the variable that tells you how much heat energy is required to change the. What is the difference between thermal. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ. Specific Heat Capacity Test Question.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula Specific Heat Capacity Test Question This topic is designed as an interactive quiz. What is the difference between thermal. If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? Test yourself on this topic. What does it mean if water has a higher specific heat capacity than oil? C = specific heat capacity, in joules. Specific Heat Capacity Test Question.
From rootsphysics.blogspot.com
Past Papers Physics OLevels and IGCSE Specific heat capacity and Specific Heat Capacity Test Question What does it mean if water has a higher specific heat capacity than oil? Test yourself on this topic. The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in. Specific Heat Capacity Test Question.
From www.studypool.com
SOLUTION 6chap heat capacity specific heat worksheet Studypool Specific Heat Capacity Test Question What is the name of the variable that tells you how much heat energy is required to change the. Specific heat capacity test questions. Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. Specific heat capacity quiz a. What is the difference between thermal. C = specific heat capacity, in joules per kilogram per degree. Specific Heat Capacity Test Question.
From zipworksheet.com
Specific Heat Worksheet Answers Specific Heat Capacity Test Question This topic is designed as an interactive quiz. What is the specific heat capacity of water? What is the name of the variable that tells you how much heat energy is required to change the. What does it mean if water has a higher specific heat capacity than oil? If something has a high specific heat capacity, why will it. Specific Heat Capacity Test Question.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube Specific Heat Capacity Test Question What does it mean if water has a higher specific heat capacity than oil? The specific heat capacity is the amount of heat energy required to raise the. What is the difference between thermal. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the. The. Specific Heat Capacity Test Question.
From studylib.net
Specific Heat Capacity Practice Problems Specific Heat Capacity Test Question Specific heat capacity quiz a. What does it mean if water has a higher specific heat capacity than oil? Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. What is the difference between thermal. Test yourself on this topic. Specific heat capacity test questions. If something has a high specific heat capacity, why will it. Specific Heat Capacity Test Question.
From ame.my.id
Calculating Specific Heat Worksheet Ame.my.id Specific Heat Capacity Test Question This topic is designed as an interactive quiz. What is the difference between thermal. Specific heat capacity test questions. The specific heat capacity is the amount of heat energy required to raise the. Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. Test yourself on this topic. If something has a high specific heat capacity,. Specific Heat Capacity Test Question.
From zipworksheet.com
Specific Heat Worksheet Answers Specific Heat Capacity Test Question What is the specific heat capacity of water? Specific heat capacity test questions. Specific heat capacity quiz a. What is the name of the variable that tells you how much heat energy is required to change the. If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? The thermal energy. Specific Heat Capacity Test Question.
From byjus.com
To Determine Specific Heat Capacity Of A Given Solid Physics Practical Specific Heat Capacity Test Question Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the. Specific heat capacity quiz a. If something has a high specific heat capacity, why will it take a lot of heat. Specific Heat Capacity Test Question.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources Specific Heat Capacity Test Question The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. Test yourself on this topic. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the. What is the difference between thermal. Specific heat capacity quiz. Specific Heat Capacity Test Question.
From teenaebboni.blogspot.com
32+ Calculate Specific Heat Capacity TeenaEbboni Specific Heat Capacity Test Question Test yourself on this topic. The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? Specific heat capacity quiz a. What is the difference between thermal. Get specific heat capacity. Specific Heat Capacity Test Question.
From studylib.net
Specific Heat Capacity Questions Specific Heat Capacity Test Question What does it mean if water has a higher specific heat capacity than oil? C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c) δ θ = change in temperature, in degrees celsius (°c) the. The specific heat capacity is the amount of heat energy required to raise the. What is the name of the variable. Specific Heat Capacity Test Question.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Specific Heat Capacity Test Question What does it mean if water has a higher specific heat capacity than oil? Specific heat capacity quiz a. Test yourself on this topic. This topic is designed as an interactive quiz. The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. What is the specific heat capacity of water?. Specific Heat Capacity Test Question.
From www.youtube.com
Specific Heat Capacity and Latent Heat Calculations YouTube Specific Heat Capacity Test Question Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. What does it mean if water has a higher specific heat capacity than oil? The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. What is the specific heat capacity of water? Specific heat capacity quiz. Specific Heat Capacity Test Question.
From www.worksheetsgo.com
Specific Heat Worksheets WorksheetsGO Specific Heat Capacity Test Question What is the difference between thermal. What is the specific heat capacity of water? Specific heat capacity test questions. Test yourself on this topic. The specific heat capacity is the amount of heat energy required to raise the. What is the name of the variable that tells you how much heat energy is required to change the. What does it. Specific Heat Capacity Test Question.
From quizlet.com
Determination of specific heat capacity of a solid Diagram Quizlet Specific Heat Capacity Test Question Test yourself on this topic. Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. This topic is designed as an interactive quiz. What is the name of the variable that tells you how much heat energy is required to change the. What is the specific heat capacity of water? Specific heat capacity test questions. Specific. Specific Heat Capacity Test Question.
From byjus.com
The SI unit of specific heat capacity of a substance is Specific Heat Capacity Test Question What is the difference between thermal. What is the specific heat capacity of water? Specific heat capacity test questions. This topic is designed as an interactive quiz. Test yourself on this topic. The specific heat capacity is the amount of heat energy required to raise the. C = specific heat capacity, in joules per kilogram per degree celsius (j/kg °c). Specific Heat Capacity Test Question.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Specific Heat Capacity Test Question If something has a high specific heat capacity, why will it take a lot of heat to change its temperature? Test yourself on this topic. The thermal energy change (gain or loss) in a substance is equal to the (amount of substance) × (shc) ×. Get specific heat capacity multiple choice questions (mcq quiz) with answers and detailed solutions. Specific. Specific Heat Capacity Test Question.