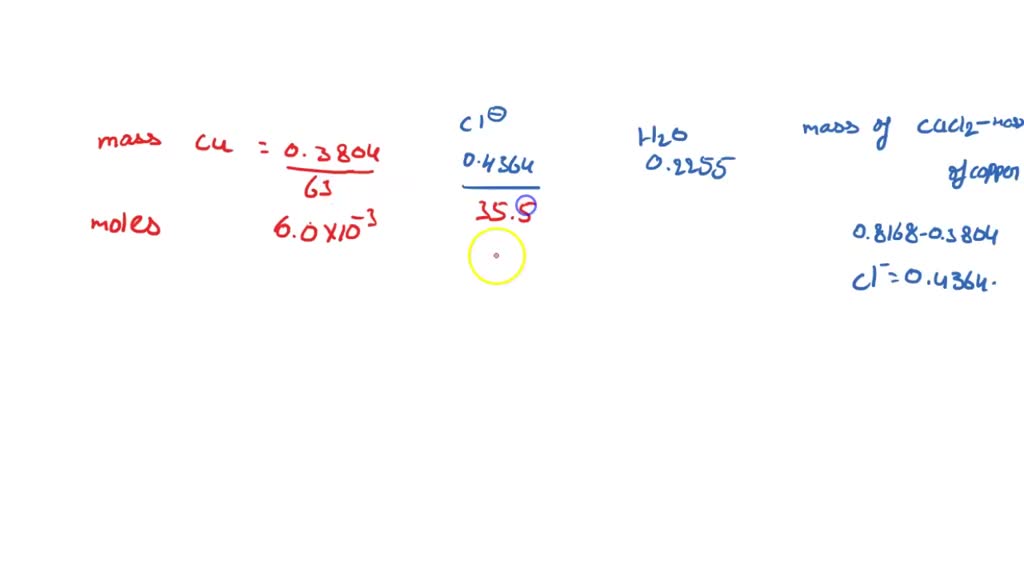Copper Chloride Hydrate Formula . Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Cucl2 + 2 naoh → cu (oh)2. A hydrate is a compound that has a specific number of water molecules within its solid structure. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. For example, in its normal state,. It is a brown solid when anhydrous, but turns a brilliant. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. See also the names and synonyms. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs:
from www.numerade.com
Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Cucl2 + 2 naoh → cu (oh)2. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: For example, in its normal state,. A hydrate is a compound that has a specific number of water molecules within its solid structure. It is a brown solid when anhydrous, but turns a brilliant. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. See also the names and synonyms.
SOLVED How many moles of water were in your sample of copper chloride
Copper Chloride Hydrate Formula Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. A hydrate is a compound that has a specific number of water molecules within its solid structure. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. It is a brown solid when anhydrous, but turns a brilliant. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: For example, in its normal state,. See also the names and synonyms. Cucl2 + 2 naoh → cu (oh)2.
From www.amazon.com
Copper (II) Chloride Dihydrate, 500g The Curated Chemical Collection Copper Chloride Hydrate Formula See also the names and synonyms. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: For example, in its normal state,. A hydrate is a compound that has a specific number of. Copper Chloride Hydrate Formula.
From www.libertysci.com
Copper(II) Chloride, dihydrate, 100 g Liberty Scientific Copper Chloride Hydrate Formula Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also.. Copper Chloride Hydrate Formula.
From lessonlibrarypate.z13.web.core.windows.net
What Are Some Examples Of Hydrates Copper Chloride Hydrate Formula A hydrate is a compound that has a specific number of water molecules within its solid structure. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Find the molecular. Copper Chloride Hydrate Formula.
From www.pinterest.com
Empirical Formula Experiment copper chloride hydrate YouTube by S Copper Chloride Hydrate Formula When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. It is. Copper Chloride Hydrate Formula.
From www.funcmater.com
Copper(II) chloride hydrate (CuCl2•XH2O )Crystals FUNCMATER Copper Chloride Hydrate Formula Cucl2 + 2 naoh → cu (oh)2. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. A hydrate is a compound that has a specific number of water molecules within its solid structure. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide. Copper Chloride Hydrate Formula.
From www.slideserve.com
PPT Determining the Empirical Formula of Copper Chloride PowerPoint Copper Chloride Hydrate Formula When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. For example, in its normal state,. Cucl2 + 2 naoh → cu (oh)2. Find the molecular formula, average mass, monoisotopic mass, chemspider. Copper Chloride Hydrate Formula.
From www.slideserve.com
PPT Determining the Empirical Formula of Copper Chloride PowerPoint Copper Chloride Hydrate Formula Cucl2 + 2 naoh → cu (oh)2. A hydrate is a compound that has a specific number of water molecules within its solid structure. It is a brown solid when anhydrous, but turns a brilliant. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: For example, in its normal state,. Find the. Copper Chloride Hydrate Formula.
From www.flinnsci.com
Copper(II) Chloride, Reagent, 100 g Flinn Scientific Copper Chloride Hydrate Formula Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. It is a brown solid when anhydrous, but turns a brilliant. When copper (ii) chloride solutions are treated with a. Copper Chloride Hydrate Formula.
From www.chegg.com
Solved The actual formula for hydrated copper chloride is Copper Chloride Hydrate Formula When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: Cucl2 + 2 naoh → cu (oh)2. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. It is a brown solid when anhydrous, but turns a brilliant. A hydrate is a compound. Copper Chloride Hydrate Formula.
From www.scribd.com
Determination of the Chemical Formula of Copper Chloride Hydrate PDF Copper Chloride Hydrate Formula It is a brown solid when anhydrous, but turns a brilliant. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Learn about the chemical formula, molecular weight, density, melting and boiling. Copper Chloride Hydrate Formula.
From www.orioner.com
Copper (II) Chloride 2hydrate, AR, 500 gm, Bendosen Copper Chloride Hydrate Formula See also the names and synonyms. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: It is a brown solid when anhydrous, but turns a brilliant. Copper (ii) chloride, also known as. Copper Chloride Hydrate Formula.
From www.youtube.com
Determining the chemical formula of copper chloride hydrate YouTube Copper Chloride Hydrate Formula Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Cucl2 + 2 naoh → cu (oh)2. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of. Copper Chloride Hydrate Formula.
From www.sciencephoto.com
Copper(II) chloride dihydrate Stock Image C027/9276 Science Photo Copper Chloride Hydrate Formula Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. See also the names and synonyms. A hydrate is a compound that has a specific number of water molecules within its solid structure. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper. Copper Chloride Hydrate Formula.
From www.chegg.com
Solved Results moles mass (g) 1.07994 Cu Cly • ZH2O Curly Copper Chloride Hydrate Formula Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: For example, in its normal state,. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the. Copper Chloride Hydrate Formula.
From www.chemkits.eu
Copper(II) chloride dihydrate, 99.5+, 10125130 Copper Chloride Hydrate Formula Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. It is a brown solid when anhydrous, but turns a brilliant. See also the names and synonyms. For example,. Copper Chloride Hydrate Formula.
From qrd-qiruide.com
Copper(II) Chloride Dihydrate Qiruide® Chemical Compounds Copper Chloride Hydrate Formula Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. A hydrate is a compound that has a specific number of water molecules within its solid structure. It is a brown solid when anhydrous, but turns a brilliant. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of. Copper Chloride Hydrate Formula.
From www.animalia-life.club
Copper Chloride Copper Chloride Hydrate Formula A hydrate is a compound that has a specific number of water molecules within its solid structure. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: It is a brown solid when anhydrous, but turns a brilliant. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards. Copper Chloride Hydrate Formula.
From www.numerade.com
SOLVED A hydrate of copper(II) chloride has the following formula Copper Chloride Hydrate Formula See also the names and synonyms. For example, in its normal state,. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. Copper(ii) chloride, also known as cupric chloride, is. Copper Chloride Hydrate Formula.
From www.numerade.com
DETERMINATION OF A CHEMICAL FORMULA EMPIRICAL FORMULA OF Copper Copper Chloride Hydrate Formula For example, in its normal state,. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. See also the names and synonyms. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. A hydrate is a compound that has a. Copper Chloride Hydrate Formula.
From www.youtube.com
Empirical Formula Experiment copper chloride hydrate YouTube Copper Chloride Hydrate Formula A hydrate is a compound that has a specific number of water molecules within its solid structure. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. For example, in its normal state,. Cucl2 + 2 naoh → cu (oh)2. When copper (ii) chloride solutions are treated with a. Copper Chloride Hydrate Formula.
From www.funcmater.com
buy Copper(II) chloride hydrate Granules manufacturers FUNCMATER Copper Chloride Hydrate Formula Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. It is a brown solid when anhydrous, but turns a brilliant. See also the names and synonyms. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. When copper. Copper Chloride Hydrate Formula.
From www.pw.live
Copper II Chloride Formula, Structure, Properties, Uses Copper Chloride Hydrate Formula Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. For example, in its normal state,. It is a brown solid when anhydrous, but turns a brilliant. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: Learn about the chemical formula,. Copper Chloride Hydrate Formula.
From www.studocu.com
Exp 4 Determination of the Chemical Formula of Copper Chloride Hydrate Copper Chloride Hydrate Formula Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl. Copper Chloride Hydrate Formula.
From worldmetalllc.com
Copper Acetate, Carbonate, Chloride, Formate & Cuprous Chloride Copper Chloride Hydrate Formula It is a brown solid when anhydrous, but turns a brilliant. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. When copper (ii) chloride solutions are treated with. Copper Chloride Hydrate Formula.
From www.scribd.com
Determining The Empirical Formula of Copper Chloride Hydrate PDF Copper Chloride Hydrate Formula It is a brown solid when anhydrous, but turns a brilliant. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. See also the names and synonyms. A hydrate. Copper Chloride Hydrate Formula.
From www.numerade.com
SOLVED How many moles of water were in your sample of copper chloride Copper Chloride Hydrate Formula Cucl2 + 2 naoh → cu (oh)2. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. A hydrate is a compound that has a specific number of water molecules. Copper Chloride Hydrate Formula.
From www.studocu.com
Exp 4 Determination of the chemical Formula of Copper Chloride Hydrate Copper Chloride Hydrate Formula It is a brown solid when anhydrous, but turns a brilliant. A hydrate is a compound that has a specific number of water molecules within its solid structure. See also the names and synonyms. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Find the molecular formula, average mass,. Copper Chloride Hydrate Formula.
From molekula.com
Purchase Copper (II) chloride dihydrate [10125130] online • Catalog Copper Chloride Hydrate Formula A hydrate is a compound that has a specific number of water molecules within its solid structure. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: See also the names and synonyms. Cucl2 + 2 naoh → cu (oh)2. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of. Copper Chloride Hydrate Formula.
From www.slideserve.com
PPT Determining the Empirical Formula of Copper Chloride PowerPoint Copper Chloride Hydrate Formula See also the names and synonyms. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with. Copper Chloride Hydrate Formula.
From studylib.net
Formula of a Copper Chloride Hydrate Copper Chloride Hydrate Formula When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: It is a brown solid when anhydrous, but turns a brilliant. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. See also the names and synonyms. A hydrate is a compound that. Copper Chloride Hydrate Formula.
From www.chegg.com
Solved 18. A student performed the same experiment you did Copper Chloride Hydrate Formula See also the names and synonyms. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. It is a brown solid when anhydrous, but turns a brilliant. A hydrate. Copper Chloride Hydrate Formula.
From www.youtube.com
Empirical Formula Lab Chemical Formula of Copper Chloride Hydrate Copper Chloride Hydrate Formula For example, in its normal state,. See also the names and synonyms. Cucl2 + 2 naoh → cu (oh)2. It is a brown solid when anhydrous, but turns a brilliant. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride dihydrate. Learn about the chemical formula, molecular weight, density, melting and. Copper Chloride Hydrate Formula.
From examquiz.netlify.app
Determining the formula of a hydrate examquiz Copper Chloride Hydrate Formula Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Copper (ii) chloride, also known as cupric chloride, is a chemical compound of copper and chlorine with the formula cucl 2. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for copper (ii) chloride. Copper Chloride Hydrate Formula.
From www.slideserve.com
PPT Formula of a Hydrate PowerPoint Presentation, free download ID Copper Chloride Hydrate Formula When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: For example, in its normal state,. Learn about the chemical formula, molecular weight, density, melting and boiling points, and health hazards of copper (ii) chloride, also. It is a brown solid when anhydrous, but turns a brilliant. Copper (ii) chloride, also known as. Copper Chloride Hydrate Formula.
From www.slideserve.com
PPT The Formula for a Hydrate PowerPoint Presentation, free download Copper Chloride Hydrate Formula Cucl2 + 2 naoh → cu (oh)2. When copper (ii) chloride solutions are treated with a base, a precipitation of copper (ii) hydroxide occurs: Copper(ii) chloride, also known as cupric chloride, is an ionic compound of copper and chlorine with the formula cucl 2. Find the molecular formula, average mass, monoisotopic mass, chemspider id, wikipedia link and other information for. Copper Chloride Hydrate Formula.