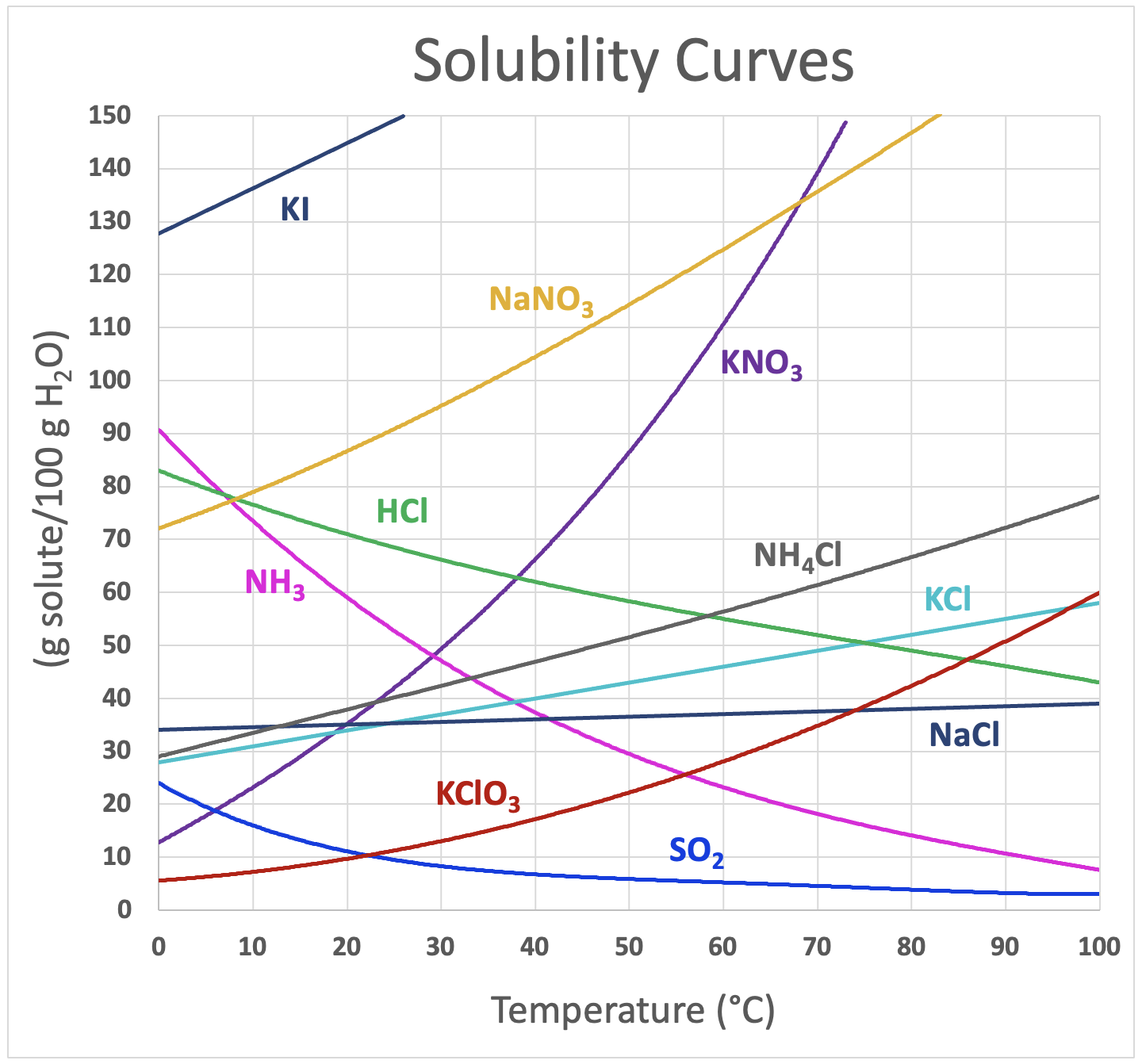
Copper Ii Hydroxide Solubility In Water . Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. More importantly, it will dissolve. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. ^ anhydrous fef3 is slightly soluble in water;. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Refer to the chart below to find reference values per gram of. Water temperature can have a significant effect on the solubility of compounds. It decomposes at temperatures above.
from mungfali.com
^ anhydrous fef3 is slightly soluble in water;. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. More importantly, it will dissolve. It decomposes at temperatures above. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values per gram of.
Solubility Chemistry Diagram
Copper Ii Hydroxide Solubility In Water Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Refer to the chart below to find reference values per gram of. ^ anhydrous fef3 is slightly soluble in water;. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. More importantly, it will dissolve. It decomposes at temperatures above. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Water temperature can have a significant effect on the solubility of compounds.
From mavink.com
Metal Solubility Chart Copper Ii Hydroxide Solubility In Water Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. More importantly,. Copper Ii Hydroxide Solubility In Water.
From www.chegg.com
Solved Calculate the solubility of copper(II) hydroxide, Copper Ii Hydroxide Solubility In Water Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Refer to the chart below to find reference values per gram of. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It decomposes at temperatures above. Water temperature can have a significant effect on the solubility. Copper Ii Hydroxide Solubility In Water.
From www.researchgate.net
Copper(II) solubility of different DIC levels compared to copper(I Copper Ii Hydroxide Solubility In Water Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. It decomposes at temperatures above. Water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values per gram. Copper Ii Hydroxide Solubility In Water.
From www.chegg.com
Solved Based on the solubility curves below, how much copper Copper Ii Hydroxide Solubility In Water Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Water temperature can have a significant effect on the solubility of compounds. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. More importantly, it will dissolve.. Copper Ii Hydroxide Solubility In Water.
From www.numerade.com
SOLVED A student measures the molar solubility of copper(II) hydroxide Copper Ii Hydroxide Solubility In Water ^ anhydrous fef3 is slightly soluble in water;. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Water temperature can have. Copper Ii Hydroxide Solubility In Water.
From www.researchgate.net
Solubility diagram for different Cu(I) and Cu(II) solids likely in new Copper Ii Hydroxide Solubility In Water Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. ^ anhydrous fef3 is slightly soluble in water;. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. It decomposes at temperatures above. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue. Copper Ii Hydroxide Solubility In Water.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper Ii Hydroxide Solubility In Water ^ anhydrous fef3 is slightly soluble in water;. Refer to the chart below to find reference values per gram of. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but. Copper Ii Hydroxide Solubility In Water.
From www.chegg.com
Solved Compare the solubility of copper(II) sulfide in each Copper Ii Hydroxide Solubility In Water More importantly, it will dissolve. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It decomposes at temperatures above. Refer. Copper Ii Hydroxide Solubility In Water.
From www.numerade.com
SOLVED A precipitate of copper(II) hydroxide is formed in a reaction Copper Ii Hydroxide Solubility In Water Water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values per gram of. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. ^ anhydrous fef3 is slightly soluble in water;. Copper(ii) hydroxide is. Copper Ii Hydroxide Solubility In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Copper Ii Hydroxide Solubility In Water Water temperature can have a significant effect on the solubility of compounds. More importantly, it will dissolve. ^ anhydrous fef3 is slightly soluble in water;. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. Refer to the chart below to find reference values per gram of. Copper(ii) hydroxide is insoluble in water under. Copper Ii Hydroxide Solubility In Water.
From ar.inspiredpencil.com
Copper Hydroxide Solution Copper Ii Hydroxide Solubility In Water ^ anhydrous fef3 is slightly soluble in water;. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Refer to the chart below to find reference values per gram. Copper Ii Hydroxide Solubility In Water.
From handwiki.org
ChemistryCopper(II) hydroxide HandWiki Copper Ii Hydroxide Solubility In Water ^ anhydrous fef3 is slightly soluble in water;. More importantly, it will dissolve. Water temperature can have a significant effect on the solubility of compounds. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Refer to the chart below to. Copper Ii Hydroxide Solubility In Water.
From www.coursehero.com
[Solved] Part A Calculate the solubility of copper(II) hydroxide (Ksp Copper Ii Hydroxide Solubility In Water Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. It decomposes at temperatures above. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. ^ anhydrous fef3 is slightly soluble in water;.. Copper Ii Hydroxide Solubility In Water.
From www.slideserve.com
PPT Matter and Change PowerPoint Presentation, free download ID9660645 Copper Ii Hydroxide Solubility In Water Refer to the chart below to find reference values per gram of. Water temperature can have a significant effect on the solubility of compounds. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. It decomposes at temperatures above. It is slightly soluble in water and more soluble in acids or ammonium. Copper Ii Hydroxide Solubility In Water.
From ar.inspiredpencil.com
Solubility Chart Chemistry 11 Copper Ii Hydroxide Solubility In Water More importantly, it will dissolve. Water temperature can have a significant effect on the solubility of compounds. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. ^ anhydrous. Copper Ii Hydroxide Solubility In Water.
From www.flinnsci.ca
Solubility Rules Chart, Notebook Size, Pad of 30 Copper Ii Hydroxide Solubility In Water Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It decomposes at temperatures above. More importantly, it will dissolve. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Refer to the chart below to find reference values per gram of. ^ anhydrous fef3 is slightly. Copper Ii Hydroxide Solubility In Water.
From www.pinterest.com
Solubility Rules Chart for Chemistry Classroom 11th chemistry Copper Ii Hydroxide Solubility In Water It decomposes at temperatures above. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Refer to the chart below to find reference values per gram of.. Copper Ii Hydroxide Solubility In Water.
From vanittaran.weebly.com
Synthesis of copper(II) hydroxide ACQUIRE Copper Ii Hydroxide Solubility In Water ^ anhydrous fef3 is slightly soluble in water;. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Refer to the chart below to find reference values per gram of. It decomposes at temperatures above. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. Copper(ii). Copper Ii Hydroxide Solubility In Water.
From www.chegg.com
Solved 10 100 8 6 Solubility of Copper Sulfate Copper Ii Hydroxide Solubility In Water Water temperature can have a significant effect on the solubility of compounds. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. It decomposes at temperatures above. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. More importantly, it will. Copper Ii Hydroxide Solubility In Water.
From www.chegg.com
Solved Consider the insoluble compound copper(II) hydroxide, Copper Ii Hydroxide Solubility In Water Refer to the chart below to find reference values per gram of. It decomposes at temperatures above. ^ anhydrous fef3 is slightly soluble in water;. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide. Copper Ii Hydroxide Solubility In Water.
From mmerevise.co.uk
Group 2 Solubility and Chemical Tests Revision MME Copper Ii Hydroxide Solubility In Water It decomposes at temperatures above. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Refer to the chart below to find reference values per gram of. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. ^ anhydrous fef3 is slightly soluble in water;. More. Copper Ii Hydroxide Solubility In Water.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Ii Hydroxide Solubility In Water Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Water temperature can have a significant effect on the solubility of compounds. It is slightly soluble in water and more. Copper Ii Hydroxide Solubility In Water.
From slideplayer.com
The Solubility Product Principle ppt download Copper Ii Hydroxide Solubility In Water True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. ^ anhydrous fef3 is slightly soluble in water;. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. More importantly, it will dissolve. Refer to. Copper Ii Hydroxide Solubility In Water.
From www.chegg.com
Solved 35. Copper(II) hydroxide, For the reaction below, Copper Ii Hydroxide Solubility In Water Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Refer to the chart below to find reference values per gram of. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. ^ anhydrous fef3 is slightly. Copper Ii Hydroxide Solubility In Water.
From www.bartleby.com
Answered Consider the insoluble compound… bartleby Copper Ii Hydroxide Solubility In Water ^ anhydrous fef3 is slightly soluble in water;. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It decomposes at temperatures above. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by. Copper Ii Hydroxide Solubility In Water.
From www.ceramic-glazes.com
Copper Hydroxide Cupric hydroxide patina for ceramics Copper Ii Hydroxide Solubility In Water It decomposes at temperatures above. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. More importantly, it will dissolve. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. ^ anhydrous fef3 is slightly. Copper Ii Hydroxide Solubility In Water.
From www.chegg.com
Solved Question 3 0/1 pts The solubility product of solid Copper Ii Hydroxide Solubility In Water More importantly, it will dissolve. Water temperature can have a significant effect on the solubility of compounds. ^ anhydrous fef3 is slightly soluble in water;. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. It decomposes at temperatures above. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution. Copper Ii Hydroxide Solubility In Water.
From www.youtube.com
Making copper hydroxide YouTube Copper Ii Hydroxide Solubility In Water It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It decomposes at temperatures above. True copper (ii) carbonate (cuco3) is rare and. Copper Ii Hydroxide Solubility In Water.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Ii Hydroxide Solubility In Water More importantly, it will dissolve. Water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values per gram of. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. ^ anhydrous fef3 is slightly soluble in water;. It decomposes at temperatures above. True copper (ii) carbonate. Copper Ii Hydroxide Solubility In Water.
From www.chegg.com
2. Copper (II) hydroxide is only very slightly Copper Ii Hydroxide Solubility In Water It decomposes at temperatures above. Refer to the chart below to find reference values per gram of. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several factors. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. True copper (ii) carbonate (cuco3) is rare and reacts with. Copper Ii Hydroxide Solubility In Water.
From ar.inspiredpencil.com
Solubility Rules Table Chemistry Copper Ii Hydroxide Solubility In Water True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. It decomposes at temperatures above. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. ^ anhydrous fef3 is slightly soluble in water;. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can be influenced by several. Copper Ii Hydroxide Solubility In Water.
From www.researchgate.net
6. Solubility versus pH curves for the thermodynamically stable Copper Ii Hydroxide Solubility In Water It decomposes at temperatures above. True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Water temperature can have a significant effect on the solubility of compounds. Refer to. Copper Ii Hydroxide Solubility In Water.
From mungfali.com
Solubility Chemistry Diagram Copper Ii Hydroxide Solubility In Water True copper (ii) carbonate (cuco3) is rare and reacts with water to form basic copper carbonate. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Refer to the chart below to find reference values per gram of. ^ anhydrous fef3 is slightly soluble in water;. Copper(ii) hydroxide is insoluble in water under normal conditions, but. Copper Ii Hydroxide Solubility In Water.
From www.numerade.com
⏩SOLVEDThe solubility of copper(II) hydroxide in water can be… Numerade Copper Ii Hydroxide Solubility In Water It decomposes at temperatures above. More importantly, it will dissolve. Refer to the chart below to find reference values per gram of. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Copper(ii) hydroxide is insoluble in water under normal conditions, but its solubility can. Copper Ii Hydroxide Solubility In Water.
From www.coursehero.com
[Solved] Part A Calculate the solubility of copper(II) hydroxide (Ksp Copper Ii Hydroxide Solubility In Water Refer to the chart below to find reference values per gram of. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Water temperature can have a significant effect on the solubility of compounds. Copper(ii) hydroxide is insoluble in water under normal conditions, but its. Copper Ii Hydroxide Solubility In Water.