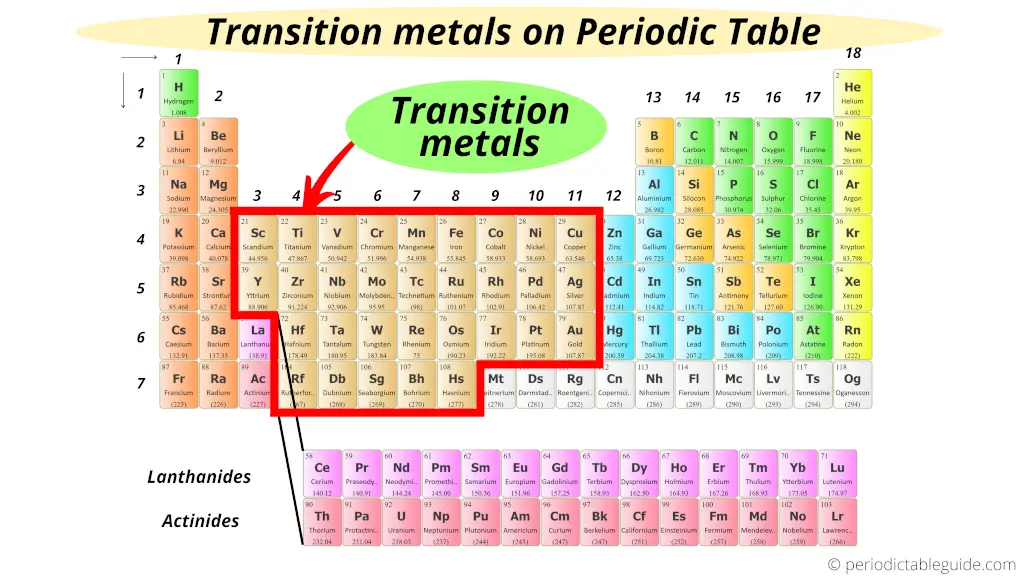
This Is A Transition Metal . The properties of individual transition metals determine which should be used for what. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. The lanthanides and the actinides at the bottom of the table are. Here is a list of elements considered transition metals or transition elements.
from periodictableguide.com
This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. The lanthanides and the actinides at the bottom of the table are. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. The properties of individual transition metals determine which should be used for what. Here is a list of elements considered transition metals or transition elements. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry.
Where are Transition Metals located on the Periodic Table?
This Is A Transition Metal This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Here is a list of elements considered transition metals or transition elements. The lanthanides and the actinides at the bottom of the table are. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The properties of individual transition metals determine which should be used for what. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital.
From studymind.co.uk
The Transition Metals (GCSE Chemistry) Study Mind This Is A Transition Metal Here is a list of elements considered transition metals or transition elements. The properties of individual transition metals determine which should be used for what. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. This page explains what a transition metal is in terms of its electronic structure,. This Is A Transition Metal.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps This Is A Transition Metal The lanthanides and the actinides at the bottom of the table are. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The properties of individual transition metals determine which should be used for what. Here is a list of elements considered transition metals or transition elements. The transition metals are the. This Is A Transition Metal.
From www.w3schools.blog
Transition metals W3schools This Is A Transition Metal These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Here is a list of elements considered transition metals or transition elements. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. This page explains what a transition metal is in. This Is A Transition Metal.
From www.haikudeck.com
Transition Metals by Sarena Kinkel This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. Here is a list of elements considered transition metals or transition elements. The properties of individual transition metals determine which should be used for what. This page explains what a transition metal is in terms of its electronic structure,. This Is A Transition Metal.
From www.slideserve.com
PPT THE TRANSITION METALS PowerPoint Presentation, free download ID This Is A Transition Metal This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The lanthanides and the actinides at the bottom of the table are. The transition. This Is A Transition Metal.
From www.slideserve.com
PPT Ch 6 review PowerPoint Presentation, free download ID2109980 This Is A Transition Metal The properties of individual transition metals determine which should be used for what. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of. This Is A Transition Metal.
From www.wisegeek.com
What Are Transition Metals? (with pictures) This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. Here is a list of elements considered transition metals or transition elements. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Transition metals and their compounds are used in a. This Is A Transition Metal.
From jonathanhenry.z13.web.core.windows.net
Transition Metal Charges Chart This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. The lanthanides and the actinides at the bottom of the table are. The properties of individual transition metals determine which should be used for what. This page explains what a transition metal is in terms of its electronic structure,. This Is A Transition Metal.
From utedzz.blogspot.com
Periodic Table Transition Metals Charges Periodic Table Timeline This Is A Transition Metal This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can. This Is A Transition Metal.
From fphoto.photoshelter.com
science elements transition metals Fundamental Photographs The Art This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. Here is a list of elements considered transition metals or transition. This Is A Transition Metal.
From www.youtube.com
Introduction to nomenclature with binary and transition metals YouTube This Is A Transition Metal These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. The lanthanides and the actinides at the bottom of the table are. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. This Is A Transition Metal.
From www.breakingatom.com
The Transition Metals This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. The lanthanides and the actinides at the bottom of the table are. The properties of individual transition metals determine which should be used for what. Transition metals and their compounds are used in a wide range of goods and. This Is A Transition Metal.
From www.priyamstudycentre.com
Transition Metals Elements, Definition, List, Properties This Is A Transition Metal The lanthanides and the actinides at the bottom of the table are. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The properties. This Is A Transition Metal.
From www.youtube.com
Transition Metals Periodic table Chemistry Khan Academy YouTube This Is A Transition Metal The lanthanides and the actinides at the bottom of the table are. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. This Is A Transition Metal.
From www.britannica.com
transition metal Definition, Properties, Elements, & Facts Britannica This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The properties of individual transition metals determine which should be used for what. Here is a list of elements considered transition. This Is A Transition Metal.
From period-faqs.com
Where Are The Transition Metals Located On The Periodic Table This Is A Transition Metal Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The transition metals. This Is A Transition Metal.
From www.tes.com
Transition metal reactions Teaching Resources This Is A Transition Metal This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. The lanthanides and the actinides at the bottom of the table are. The properties of individual transition metals determine which should be used for what. Here is a list of elements considered. This Is A Transition Metal.
From app.jove.com
Transition Metals Electron Configurations and Properties Concept This Is A Transition Metal Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The properties of individual transition metals determine which should be used for what. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. Here is a list of elements considered transition. This Is A Transition Metal.
From www.slideserve.com
PPT Transition Metals PowerPoint Presentation, free download ID6736264 This Is A Transition Metal Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. The lanthanides and the actinides at the bottom of the table are. The properties of individual transition metals determine which should be used for what. The transition metals are the metallic elements that serve as a bridge, or transition, between the two. This Is A Transition Metal.
From periodictableguide.com
Where are Transition Metals located on the Periodic Table? This Is A Transition Metal The properties of individual transition metals determine which should be used for what. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. These elements are called transition metals because electrons in their atoms. This Is A Transition Metal.
From newtondesk.com
Transition Metals On The Periodic Table An Overview NewtonDesk This Is A Transition Metal The properties of individual transition metals determine which should be used for what. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. The lanthanides and the actinides at the bottom of the table are. These elements are called transition metals because electrons in their atoms transition to fill the d subshell. This Is A Transition Metal.
From www.slideserve.com
PPT Transition Metals & Coordination Chemistry PowerPoint This Is A Transition Metal These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Here is a list of elements considered transition metals or transition elements. The lanthanides and the actinides at the bottom of the table are. The transition metals are the metallic elements that serve as a bridge, or transition, between. This Is A Transition Metal.
From www.youtube.com
Lecture 28 Transition Metals and Transition Metal Complexes YouTube This Is A Transition Metal This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. The lanthanides and the actinides at the bottom of the table. This Is A Transition Metal.
From sciencenotes.org
Transition Metals Definition, List and Properties This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry.. This Is A Transition Metal.
From www.slideserve.com
PPT Transitionmetal Organometallics PowerPoint Presentation, free This Is A Transition Metal Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. The properties of individual transition metals determine which should be used for what. The lanthanides and the actinides at the bottom of the table are. The transition metals are the metallic elements that serve as a bridge, or transition, between the two. This Is A Transition Metal.
From www.sliderbase.com
Element Classes Presentation Chemistry This Is A Transition Metal Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features. This Is A Transition Metal.
From knordslearning.com
Inner Transition Metals Periodic Table (With Images) This Is A Transition Metal Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. The properties of. This Is A Transition Metal.
From www.slideserve.com
PPT “Transition” metals PowerPoint Presentation, free download ID This Is A Transition Metal This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. These elements are called transition metals because electrons in their atoms. This Is A Transition Metal.
From tableformeonly.blogspot.com
What Are The Transition Metals On The Periodic Table This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. Here is a list of elements considered transition metals or transition elements. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal. This Is A Transition Metal.
From studylib.net
Chapter_23_Transition_Metal_Chemistry This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. Here is a list of elements considered transition metals or transition. This Is A Transition Metal.
From www.slideserve.com
PPT Transition Metals PowerPoint Presentation, free download ID2275684 This Is A Transition Metal This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition metal chemistry. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The lanthanides and the actinides at the bottom of the table are. The properties. This Is A Transition Metal.
From www.pearson.com
Transition Metals Periodic table Chemistry Khan Academy This Is A Transition Metal The properties of individual transition metals determine which should be used for what. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. This page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of transition. This Is A Transition Metal.
From www.youtube.com
The Transition Metals YouTube This Is A Transition Metal Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. These elements are called transition metals because electrons in their atoms transition to fill the d subshell or d sublevel orbital. The lanthanides and the actinides at the bottom of the table are. The properties of individual transition metals determine which should. This Is A Transition Metal.
From knordslearning.com
Transition Metals Periodic Table (With Images) This Is A Transition Metal The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Here is a. This Is A Transition Metal.
From www.vedantu.com
Uses of Transition Metals Learn Important Terms and Concepts This Is A Transition Metal The properties of individual transition metals determine which should be used for what. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. Transition metals and their compounds are used in. This Is A Transition Metal.