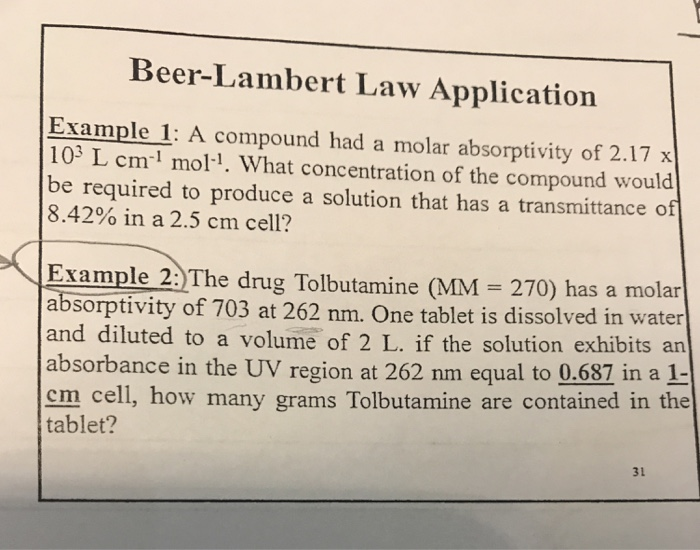
Beer Lambert Law Practice Problems . It states that, for a given material, the sample path length and. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. What will be the units of ∈, the specific.
from www.chegg.com
[1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It states that, for a given material, the sample path length and. What will be the units of ∈, the specific.
Solved BeerLambert Law Application Example 1 A compound
Beer Lambert Law Practice Problems [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. It states that, for a given material, the sample path length and. What will be the units of ∈, the specific.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer Lambert Law Practice Problems In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It states that, for a given material, the sample path length and. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. What will. Beer Lambert Law Practice Problems.
From www.numerade.com
SOLVEDThe BeerLambert Law. A beam of light enters a medium such as Beer Lambert Law Practice Problems It states that, for a given material, the sample path length and. What will be the units of ∈, the specific. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore beer's law by creating colorful solutions and measuring light. Beer Lambert Law Practice Problems.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Practice Problems In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. What will be the units of ∈, the specific. It states that, for a given material, the sample path length and. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From www.youtube.com
Absorbance Transmittance Numerical Practice problem on Lambert Beer Beer Lambert Law Practice Problems What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It states that, for a given material, the sample path length and. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light. Beer Lambert Law Practice Problems.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law Practice Problems Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It states that, for a. Beer Lambert Law Practice Problems.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law Beer Lambert Law Practice Problems [1] the absorbance is a dimensionless quantity, that is, a quantity without units. What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It states that, for a. Beer Lambert Law Practice Problems.
From www.chegg.com
Solved BeerLambert law states the absorbance of a solution Beer Lambert Law Practice Problems It states that, for a given material, the sample path length and. What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From www.studocu.com
3.13 BeerLambert Law Practice Problems 3 BeerLambert Law Practice Beer Lambert Law Practice Problems What will be the units of ∈, the specific. It states that, for a given material, the sample path length and. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light. Beer Lambert Law Practice Problems.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert Law Practice Problems It states that, for a given material, the sample path length and. What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From www.youtube.com
Solution of a problem based on BeerLambert Law YouTube Beer Lambert Law Practice Problems What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It states that, for a. Beer Lambert Law Practice Problems.
From www.studocu.com
Biochemistry Few Questions and answers based on BeerLambert Law and Beer Lambert Law Practice Problems What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a. Beer Lambert Law Practice Problems.
From www.coursehero.com
[Solved] What is the LambertBeer Law and how can you use it to Beer Lambert Law Practice Problems What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. It states that, for a. Beer Lambert Law Practice Problems.
From www.slideshare.net
Beer Lambert Law solved problems Beer Lambert Law Practice Problems Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. What will be the units of ∈, the specific. It states that, for a. Beer Lambert Law Practice Problems.
From www.studypool.com
SOLUTION 2 beer lambert law complete Studypool Beer Lambert Law Practice Problems It states that, for a given material, the sample path length and. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. What will be the units of ∈, the specific. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law Practice Problems [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a. Beer Lambert Law Practice Problems.
From www.slideshare.net
Beer Lambert Law solved problems Beer Lambert Law Practice Problems What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a given material, the sample path length and. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From www.chegg.com
Solved BeerLambert Law Application Example 1 A compound Beer Lambert Law Practice Problems What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a given material, the sample path length and. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length. Beer Lambert Law Practice Problems.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Practice Problems [1] the absorbance is a dimensionless quantity, that is, a quantity without units. It states that, for a given material, the sample path length and. What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length. Beer Lambert Law Practice Problems.
From www.studocu.com
Beer lamberts law problem practice question engineering chemistry The Beer Lambert Law Practice Problems [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a given material, the sample path length and. What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length. Beer Lambert Law Practice Problems.
From app.formative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Beer Lambert Law Practice Problems It states that, for a given material, the sample path length and. What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From www.studypool.com
SOLUTION Beer lambert law uv visible practice examples woodward fieser Beer Lambert Law Practice Problems It states that, for a given material, the sample path length and. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From www.chegg.com
Solved The BeerLambert Law is useful for determining the Beer Lambert Law Practice Problems Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a given material, the sample path length and. What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer Lambert Law Practice Problems In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. It states that, for a given material, the sample path length and. What will. Beer Lambert Law Practice Problems.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law Practice Problems Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. It states that, for a given material, the sample path length and. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. What will. Beer Lambert Law Practice Problems.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Practice Problems In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a given material, the sample path length and. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From www.chegg.com
Solved The BeerLambert law relates the absorbance A of a Beer Lambert Law Practice Problems [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. What will be the units of ∈, the specific. It states that, for a. Beer Lambert Law Practice Problems.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer Lambert Law Practice Problems It states that, for a given material, the sample path length and. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. What will. Beer Lambert Law Practice Problems.
From www.studypool.com
SOLUTION Beer lambert law uv visible practice examples woodward fieser Beer Lambert Law Practice Problems Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a given material, the sample path length and. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. What will be the units of ∈, the specific. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From www.slideshare.net
Beer Lambert Law solved problems Beer Lambert Law Practice Problems Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It states that, for a given material, the sample path length and. What will. Beer Lambert Law Practice Problems.
From www.studypool.com
SOLUTION Beer lambert law Studypool Beer Lambert Law Practice Problems In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a. Beer Lambert Law Practice Problems.
From www.slideshare.net
Beer Lambert Law solved problems Beer Lambert Law Practice Problems Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a given material, the sample path length and. What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless. Beer Lambert Law Practice Problems.
From study.com
How to Find the Path Length Using the BeerLambert Law Chemistry Beer Lambert Law Practice Problems Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. What will be the units of ∈, the specific. It states that, for a given material, the sample path length and. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length. Beer Lambert Law Practice Problems.
From egpat.com
Deviations from BeerLambert law Beer Lambert Law Practice Problems [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a given material, the sample path length and. What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length. Beer Lambert Law Practice Problems.
From www.slideshare.net
Beer Lambert Law solved problems Beer Lambert Law Practice Problems It states that, for a given material, the sample path length and. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore beer's law by creating colorful solutions and measuring light. Beer Lambert Law Practice Problems.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law Practice Problems [1] the absorbance is a dimensionless quantity, that is, a quantity without units. What will be the units of ∈, the specific. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It states that, for a given material, the sample path length and. In most experiments, molar absorptivity (ε) and the length. Beer Lambert Law Practice Problems.