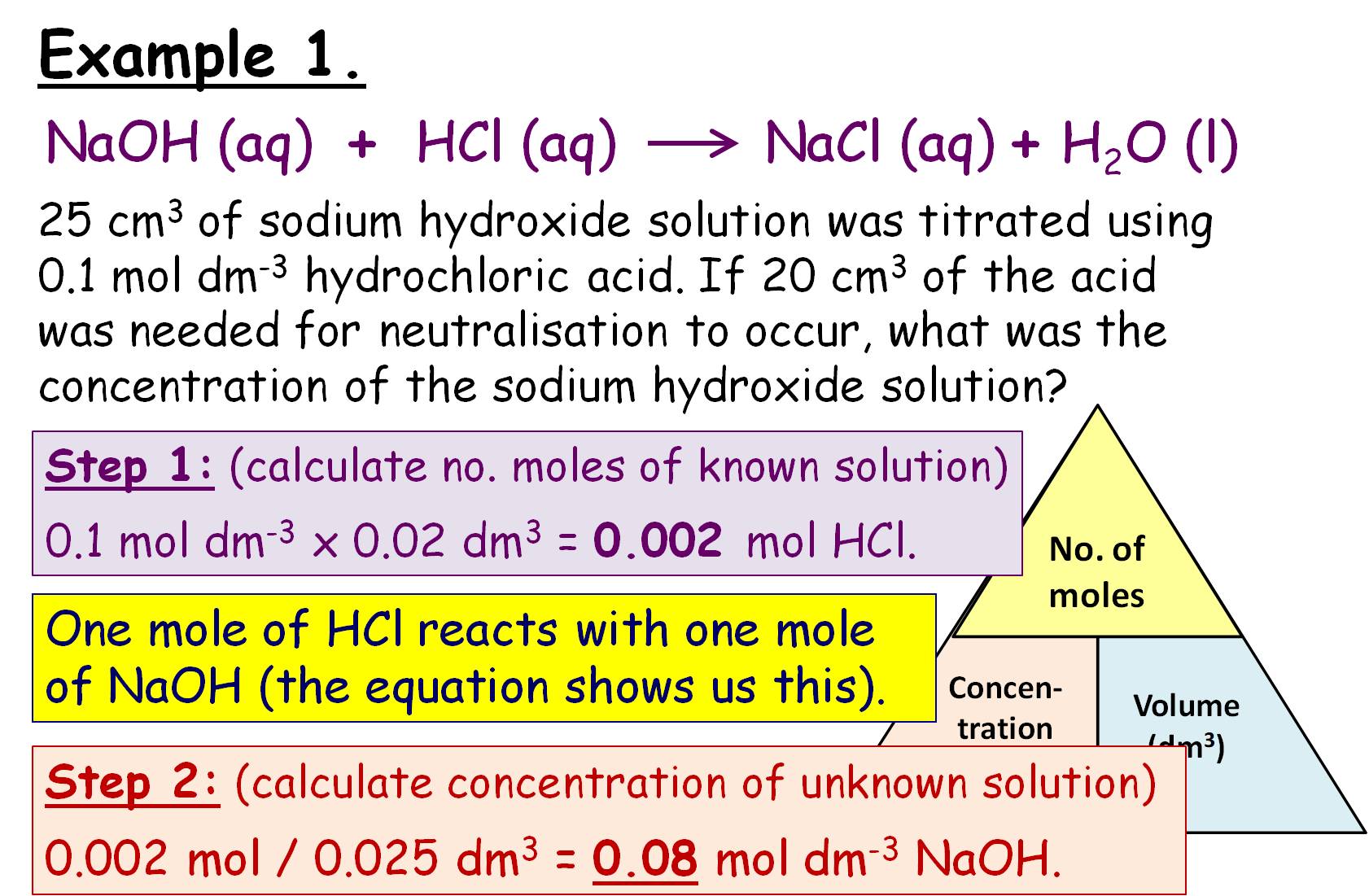
Titration Gcse Cognito . Titration is a method used to prepare salts if the reactants are soluble. They can determine exactly how much alkali is. Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Part of chemistry (single science). Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Titrations are a method of analysing the concentration of solutions. The equipment used in a titration ; The steps in carrying out a titration; What should you do for the first titration and why? This required practical involves using. Answer in your head, out. Concentration and volumes of reactants can be calculated from titrations. A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa).
from www.tes.com
They can determine exactly how much alkali is. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). Titrations are a method of analysing the concentration of solutions. Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Titration is a method used to prepare salts if the reactants are soluble. What should you do for the first titration and why? This required practical involves using. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Answer in your head, out. The equipment used in a titration ;
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d
Titration Gcse Cognito Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Concentration and volumes of reactants can be calculated from titrations. The steps in carrying out a titration; This required practical involves using. Answer in your head, out. Part of chemistry (single science). They can determine exactly how much alkali is. The equipment used in a titration ; Titrations are a method of analysing the concentration of solutions. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Titration is a method used to prepare salts if the reactants are soluble. A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. What should you do for the first titration and why? Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration Gcse Cognito A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). Concentration and volumes of reactants can be calculated from titrations. Part of chemistry (single science). Answer in your head, out. A titration is a technique to accurately measure the volume of acid needed to neutralise an. Titration Gcse Cognito.
From revisechemistry.uk
Titration OCR GCSE PAG 6 revisechemistry.uk Titration Gcse Cognito The equipment used in a titration ; Concentration and volumes of reactants can be calculated from titrations. Answer in your head, out. A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. What should you do for the first titration and why? This required practical involves using. Titrations are a method of analysing. Titration Gcse Cognito.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Gcse Cognito What should you do for the first titration and why? They can determine exactly how much alkali is. Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. The steps in carrying out a titration; Answer in your head, out.. Titration Gcse Cognito.
From www.youtube.com
What is a titration (GCSE Chemistry) YouTube Titration Gcse Cognito The steps in carrying out a titration; Answer in your head, out. Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. This required practical involves using. What should you do for the first titration and why? A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an. Titration Gcse Cognito.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Titration Gcse Cognito A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. Titrations are a method of analysing the concentration of solutions. Part of chemistry (single science). Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. The steps in carrying out a titration; A titration is a. Titration Gcse Cognito.
From www.youtube.com
Cognito platform GCSE Physics Transverse & Longitudinal Waves YouTube Titration Gcse Cognito The steps in carrying out a titration; They can determine exactly how much alkali is. The equipment used in a titration ; Part of chemistry (single science). Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Answer in your head, out. Concentration and volumes of reactants can be calculated from titrations. This. Titration Gcse Cognito.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Titration Gcse Cognito What should you do for the first titration and why? This required practical involves using. Titrations are a method of analysing the concentration of solutions. Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Titration is a method used to prepare salts if the reactants are soluble. Answer in your head, out.. Titration Gcse Cognito.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Titration Gcse Cognito Titration is a method used to prepare salts if the reactants are soluble. Answer in your head, out. The steps in carrying out a titration; This required practical involves using. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). Do a rough titration to get. Titration Gcse Cognito.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Gcse Cognito Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Titration is a method used to prepare salts if the reactants are soluble. This required practical involves using. Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Concentration and volumes of reactants can be calculated from titrations. Do a. Titration Gcse Cognito.
From www.savemyexams.com
Titrations OCR Gateway GCSE Chemistry Revision Notes 2018 Titration Gcse Cognito The equipment used in a titration ; This required practical involves using. What should you do for the first titration and why? Concentration and volumes of reactants can be calculated from titrations. The steps in carrying out a titration; Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Do a rough titration to get an approximate. Titration Gcse Cognito.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Gcse Cognito Concentration and volumes of reactants can be calculated from titrations. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). This required practical involves using. Answer in your head, out. A titration is a technique to accurately measure the volume of acid needed to neutralise an. Titration Gcse Cognito.
From www.savemyexams.com
Required Practical Strong Acid & Strong Alkali Titration AQA GCSE Titration Gcse Cognito Titrations are a method of analysing the concentration of solutions. What should you do for the first titration and why? They can determine exactly how much alkali is. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). This required practical involves using. Answer in your. Titration Gcse Cognito.
From www.youtube.com
Titration Calculation Exam Question Walkthrough|AQA GCSE Chemistry Titration Gcse Cognito A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. Part of chemistry (single science). Concentration and volumes of reactants can be calculated from titrations. They can determine exactly how much alkali is. This required practical involves using. Determination of the reacting volumes of solutions of a strong acid and a strong alkali. Titration Gcse Cognito.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Gcse Cognito Answer in your head, out. Titrations are a method of analysing the concentration of solutions. This required practical involves using. Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Concentration and volumes of reactants can be calculated from titrations. They can determine exactly how much alkali is. A titration is a practical. Titration Gcse Cognito.
From cognitoedu.org
Cognito Learn GCSE Maths, Biology, Physics and Chemistry Completely Titration Gcse Cognito Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Part of chemistry (single science). The steps in carrying out a titration; A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. They can determine exactly how much alkali is. What should you do for the. Titration Gcse Cognito.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Titration Gcse Cognito Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. This required practical involves using. Titrations are a method of analysing the concentration of solutions. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). Titration is a method used to prepare salts. Titration Gcse Cognito.
From www.youtube.com
Titration for AQA 91 GCSE Chemistry (Separate Science) YouTube Titration Gcse Cognito The equipment used in a titration ; What should you do for the first titration and why? Answer in your head, out. Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. They can determine exactly how much alkali is. The steps in carrying out a titration; Part of chemistry (single science). Concentration. Titration Gcse Cognito.
From www.studypool.com
SOLUTION Chemsheets gcse 1105 titrations 1 ans 93ghs Studypool Titration Gcse Cognito Answer in your head, out. The equipment used in a titration ; A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. They can determine exactly how much alkali is. Comprehensive quiz on titration practical for. Titration Gcse Cognito.
From www.scribd.com
GCSE Chemistry Titrations Questions With Answers PDF Chemistry Titration Gcse Cognito Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. This required practical involves using. The equipment used in a titration ; A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. Answer in your head, out. A titration is a practical technique that allows us to accurately measure how. Titration Gcse Cognito.
From www.youtube.com
Neutralisation (Titrations) AQA GCSE Required Practical YouTube Titration Gcse Cognito Part of chemistry (single science). Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The steps in carrying out a titration; Titrations are a method of analysing the concentration of solutions. Answer in your head, out. Concentration and volumes of reactants can be calculated from titrations. What should you do for the. Titration Gcse Cognito.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Gcse Cognito A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). Concentration and volumes of reactants can be calculated from titrations. The steps in carrying out a titration; This required practical involves using. Do a rough titration to get an approximate idea of the volume required for. Titration Gcse Cognito.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration Gcse Cognito Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. The equipment used in a titration ; This required practical involves using. Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Titrations are a method of analysing the concentration of solutions. What should you do for the first titration. Titration Gcse Cognito.
From www.oakabooks.co.uk
KS4/GCSE Chemical Titrations Revision Resource For Dyslexics Titration Gcse Cognito Answer in your head, out. Concentration and volumes of reactants can be calculated from titrations. The equipment used in a titration ; Titrations are a method of analysing the concentration of solutions. Titration is a method used to prepare salts if the reactants are soluble. Do a rough titration to get an approximate idea of the volume required for neutralisation. Titration Gcse Cognito.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Gcse Cognito Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Part of chemistry (single science). The steps in carrying out a titration; Answer in your head, out. The equipment used in a titration ; What should. Titration Gcse Cognito.
From www.youtube.com
Titrations Titration Calculations GCSE Chemistry Explained YouTube Titration Gcse Cognito The equipment used in a titration ; A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Titration is a method used to prepare salts if the reactants are soluble. They can determine exactly how much alkali is. Part of. Titration Gcse Cognito.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Titration Gcse Cognito Answer in your head, out. What should you do for the first titration and why? The steps in carrying out a titration; Concentration and volumes of reactants can be calculated from titrations. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). A titration is a. Titration Gcse Cognito.
From www.studypool.com
SOLUTION Chemsheets gcse 1105 titrations 1 yk this is just trash Titration Gcse Cognito They can determine exactly how much alkali is. The equipment used in a titration ; Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Determination of the reacting volumes of solutions of a strong acid and a strong alkali. Titration Gcse Cognito.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration Gcse Cognito The steps in carrying out a titration; Answer in your head, out. Concentration and volumes of reactants can be calculated from titrations. Do a rough titration to get an approximate idea of the volume required for neutralisation (the end. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Titration is a method. Titration Gcse Cognito.
From www.youtube.com
GCSE Daily Revision Task 41 Titrations YouTube Titration Gcse Cognito Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. What should you do for the first titration and why? Part of chemistry (single science). Titrations are a method of analysing the concentration of solutions. They can determine exactly how much alkali is. This required practical involves using. Concentration and volumes of reactants. Titration Gcse Cognito.
From www.bbc.co.uk
Method 8. Titration GCSE Chemistry (Single Science) Revision CCEA Titration Gcse Cognito Titrations are a method of analysing the concentration of solutions. A titration is a practical technique that allows us to accurately measure how much acid is required to neutralise an alkali (or vice versa). They can determine exactly how much alkali is. What should you do for the first titration and why? Titration is a method used to prepare salts. Titration Gcse Cognito.
From rocketsheets.co.uk
Titrations Home Learning Worksheet GCSE rocketsheets.co.uk Titration Gcse Cognito Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Titration is a method used to prepare salts if the reactants are soluble. They can determine exactly how much alkali is. Titrations are a method of analysing the concentration of solutions. The equipment used in a titration ; Do a rough titration to get an approximate idea. Titration Gcse Cognito.
From www.tes.com
AQA GCSE chemistry Unit 4 Lesson 6 Acid and Alkali Titrations and Titration Gcse Cognito A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. Titration is a method used to prepare salts if the reactants are soluble. Concentration and volumes of reactants can be calculated from titrations. The steps in carrying out a titration; Do a rough titration to get an approximate idea of the volume required. Titration Gcse Cognito.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Titration Gcse Cognito Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. This required practical involves using. Do a rough titration to get an approximate. Titration Gcse Cognito.
From www.vrogue.co
Chemistry Notes Igcse Gcse Olevel Titration Igcse Gcs vrogue.co Titration Gcse Cognito Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. What should you do for the first titration and why? A titration is a technique to accurately measure the volume of acid needed to neutralise an alkali. Part of chemistry (single science). Titrations are a method of analysing the concentration of solutions. Comprehensive. Titration Gcse Cognito.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Gcse Cognito Comprehensive quiz on titration practical for the gcse chemistry edexcel igcse triple specification. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Part of chemistry (single science). The equipment used in a titration ; Answer in your head, out. They can determine exactly how much alkali is. The steps in carrying out. Titration Gcse Cognito.