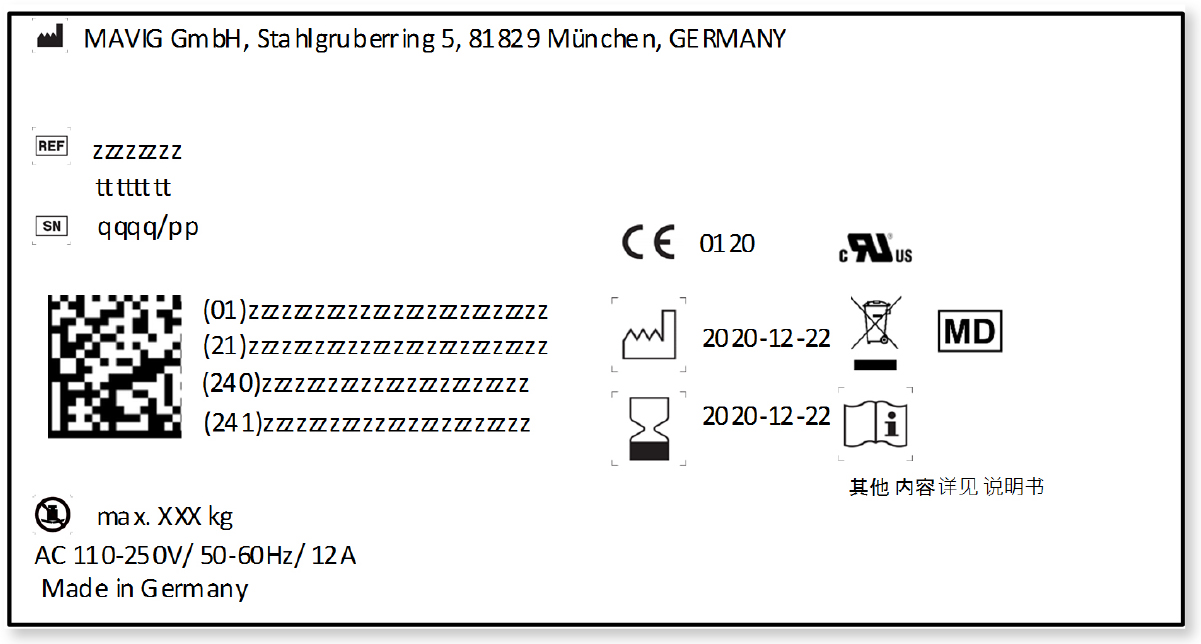
Labeling Medical Devices Standard . Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Various sections of the qs regulation have an impact on labeling:
from mavig.com
Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have an impact on labeling: This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier.
New Product Labeling due to MDR MAVIG
Labeling Medical Devices Standard This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have an impact on labeling:
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Labeling Medical Devices Standard Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices. Labeling Medical Devices Standard.
From mavink.com
Medical Device Labeling Symbols Labeling Medical Devices Standard Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Labeling Medical Devices Standard.
From mavig.com
New Product Labeling due to MDR MAVIG Labeling Medical Devices Standard This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Labeling Medical Devices Standard.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Labeling Medical Devices Standard.
From www.flexo-graphics.com
Medical Device Labeling Standards Best Medical Labels Labeling Medical Devices Standard Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have an impact on labeling: This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Labeling Medical Devices Standard.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Labeling Medical Devices Standard Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices. Labeling Medical Devices Standard.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Labeling Medical Devices Standard.
From www.alamy.com
Full set of medical device packaging symbols with warning information Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From mavink.com
Medical Device Labeling Symbols Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From mavink.com
Medical Device Labeling Symbols Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. This guidance document describes the general labelling principles for medical devices and ivd medical devices. Labeling Medical Devices Standard.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Labeling Medical Devices Standard This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Labeling Medical Devices Standard.
From trovoadasonhos.blogspot.com
Medical Device Label Symbols Trovoadasonhos Labeling Medical Devices Standard Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labeling Medical Devices Standard This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have. Labeling Medical Devices Standard.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. This guidance document describes the general labelling principles for medical devices and ivd medical devices. Labeling Medical Devices Standard.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labeling Medical Devices Standard Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have. Labeling Medical Devices Standard.
From www.linkedin.com
Introduction to Medical Device Labeling Labeling Medical Devices Standard This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From blogs.sw.siemens.com
Siemens PLM for Medical Devices Labeling and UDI solution Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices. Labeling Medical Devices Standard.
From clin-r.com
Labels for Medical Devices Clin R Labeling Medical Devices Standard Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have. Labeling Medical Devices Standard.
From lynnandmikelbaby.blogspot.com
Medical Device Label Symbols Best Label Ideas 2019 Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Labeling Medical Devices Standard This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Various sections of the qs regulation have. Labeling Medical Devices Standard.
From www.extremitymedical.com
Quality and Regulatory Extremity Medical Labeling Medical Devices Standard This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Labeling Medical Devices Standard.
From nextplus.io
Medical Device Labeling Compliant & UserFriendly Guide Next Plus Labeling Medical Devices Standard This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From clin-r.com
Labels for Medical Devices Clin R Labeling Medical Devices Standard Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From ectcolllefi.cf
Iso 15223 1 2012 Medical Devices symbols to be Used with Medical device Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices. Labeling Medical Devices Standard.
From www.barcode-us.com
Medical Devices UDI Labeling Medical Devices Standard Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. This guidance document describes the general labelling principles for medical devices and ivd medical devices. Labeling Medical Devices Standard.
From abr.com
Label Compliance AB&R® (American Barcode and RFID) Labeling Medical Devices Standard Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Labeling Medical Devices Standard Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Various sections of the qs regulation have an impact on labeling: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices. Labeling Medical Devices Standard.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labeling Medical Devices Standard Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection. Labeling Medical Devices Standard.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Labeling Medical Devices Standard This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Section 21 cfr 820.80 (b) requires the inspection and testing of incoming. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Various sections of the qs regulation have. Labeling Medical Devices Standard.