Cl + Kbr Ionic Equation . Chlorine + potassium iodide → potassium chloride + iodine. The balanced equation will be calculated along. Net ionic equations show only the. enter an equation of an ionic chemical equation and press the balance button. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. write net ionic equations for chemical reactions between ionic compounds. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. a solution of chlorine can displace iodine from potassium iodide solution: complete ionic equations show dissolved ionic solids as separated ions. Cl 2 (aq) + 2ki (aq).
from www.numerade.com
enter an equation of an ionic chemical equation and press the balance button. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. a solution of chlorine can displace iodine from potassium iodide solution: a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. Cl 2 (aq) + 2ki (aq). Net ionic equations show only the. The balanced equation will be calculated along. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. complete ionic equations show dissolved ionic solids as separated ions. Chlorine + potassium iodide → potassium chloride + iodine.
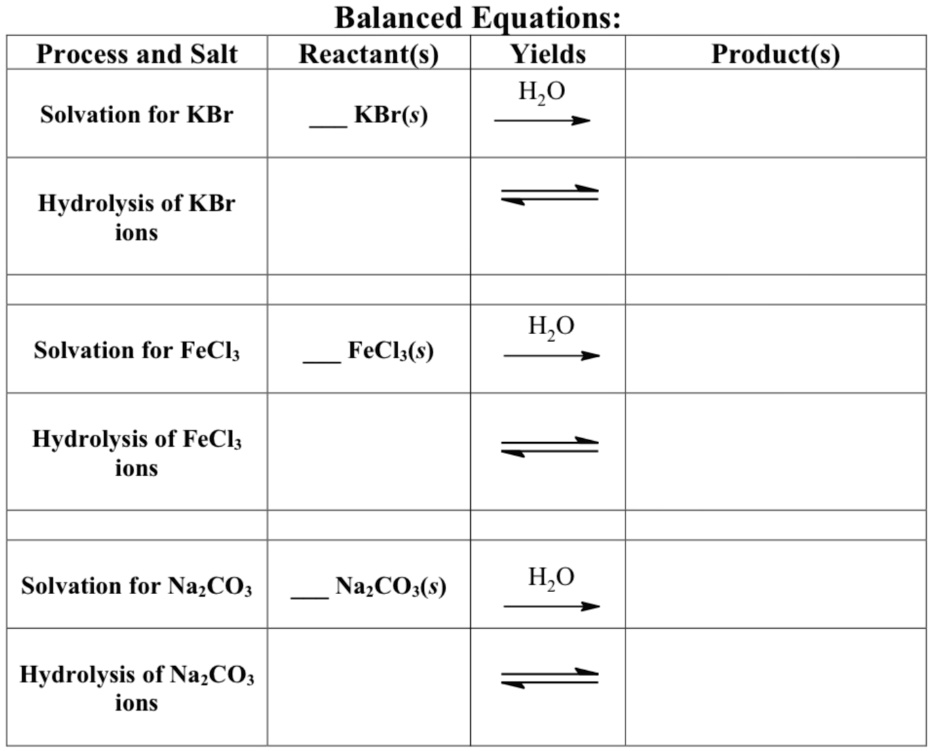
SOLVED Balanced Equations Reactant(s) Yields H2O + KBr(s) Process and
Cl + Kbr Ionic Equation Chlorine + potassium iodide → potassium chloride + iodine. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. Chlorine + potassium iodide → potassium chloride + iodine. a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. enter an equation of an ionic chemical equation and press the balance button. a solution of chlorine can displace iodine from potassium iodide solution: The balanced equation will be calculated along. complete ionic equations show dissolved ionic solids as separated ions. write net ionic equations for chemical reactions between ionic compounds. Cl 2 (aq) + 2ki (aq). Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. Net ionic equations show only the.
From www.numerade.com
SOLVED Draw a Lewis diagram to show the formation of the ionic Cl + Kbr Ionic Equation write net ionic equations for chemical reactions between ionic compounds. Cl 2 (aq) + 2ki (aq). Chlorine + potassium iodide → potassium chloride + iodine. a solution of chlorine can displace iodine from potassium iodide solution: enter an equation of an ionic chemical equation and press the balance button. Net ionic equations show only the. The balanced. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED Write a balanced net ionic equation for the reaction of AgNO3 Cl + Kbr Ionic Equation Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. . Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVEDIn the ionic compounds LiF, NaCl, KBr, and RbI, the measured Cl + Kbr Ionic Equation enter an equation of an ionic chemical equation and press the balance button. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. Chlorine + potassium iodide → potassium chloride + iodine. write net ionic equations for chemical reactions between. Cl + Kbr Ionic Equation.
From www.pearson.com
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cat Cl + Kbr Ionic Equation The balanced equation will be calculated along. write net ionic equations for chemical reactions between ionic compounds. Cl 2 (aq) + 2ki (aq). complete ionic equations show dissolved ionic solids as separated ions. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced. Cl + Kbr Ionic Equation.
From slideplayer.com
Chapter 11 Chemical Reactions. ppt download Cl + Kbr Ionic Equation Net ionic equations show only the. complete ionic equations show dissolved ionic solids as separated ions. The balanced equation will be calculated along. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. Kbr (s) → k +. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED Write balanced complete chemical equation the complete ionic Cl + Kbr Ionic Equation write net ionic equations for chemical reactions between ionic compounds. enter an equation of an ionic chemical equation and press the balance button. complete ionic equations show dissolved ionic solids as separated ions. a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. a solution of chlorine. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED Observation of Halogens Halides Salt Solution KCl, KBr, Cl Cl + Kbr Ionic Equation Cl 2 (aq) + 2ki (aq). 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. complete ionic equations show dissolved ionic solids as separated ions. The balanced equation will be calculated along. a complete ionic equation. Cl + Kbr Ionic Equation.
From slideplayer.com
Ionic Compounds Writing Formulas ppt download Cl + Kbr Ionic Equation enter an equation of an ionic chemical equation and press the balance button. complete ionic equations show dissolved ionic solids as separated ions. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. Chlorine + potassium iodide → potassium chloride. Cl + Kbr Ionic Equation.
From www.slideserve.com
PPT Solubility and Net Ionic Equations PowerPoint Presentation, free Cl + Kbr Ionic Equation write net ionic equations for chemical reactions between ionic compounds. a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. Net ionic equations show only the. Chlorine + potassium iodide → potassium chloride + iodine. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED in a solution containing 0.O1OM NazSOa and 0.O20M What would Cl + Kbr Ionic Equation write net ionic equations for chemical reactions between ionic compounds. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as.. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED Chapter 4 Worksheet Balance the following chemical equations Cl + Kbr Ionic Equation Cl 2 (aq) + 2ki (aq). 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. a solution of chlorine can displace iodine from potassium iodide solution: Kbr (s) → k + (aq) + br − (aq) not. Cl + Kbr Ionic Equation.
From www.slideserve.com
PPT Ionic Equations.... PowerPoint Presentation, free download ID Cl + Kbr Ionic Equation a solution of chlorine can displace iodine from potassium iodide solution: The balanced equation will be calculated along. complete ionic equations show dissolved ionic solids as separated ions. Net ionic equations show only the. enter an equation of an ionic chemical equation and press the balance button. Chlorine + potassium iodide → potassium chloride + iodine. Kbr. Cl + Kbr Ionic Equation.
From www.youtube.com
KBr Ionic Bonding YouTube Cl + Kbr Ionic Equation write net ionic equations for chemical reactions between ionic compounds. a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. enter an equation of an ionic chemical equation and press the balance button. a solution of chlorine can displace iodine from potassium iodide solution: The balanced equation will. Cl + Kbr Ionic Equation.
From www.chegg.com
Solved Balance the following equation KBr (aq) + Cl2 (aq) Cl + Kbr Ionic Equation write net ionic equations for chemical reactions between ionic compounds. enter an equation of an ionic chemical equation and press the balance button. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. Chlorine + potassium iodide → potassium chloride. Cl + Kbr Ionic Equation.
From byjus.com
the limiting ionic conduc†an ces of NaCl , KCl and KBr are 126.5 ,150 Cl + Kbr Ionic Equation The balanced equation will be calculated along. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. enter an equation of an ionic chemical equation and press the balance button. Cl 2 (aq) + 2ki (aq). Kbr (s). Cl + Kbr Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for AgNO3 + KBr = KNO3 + AgBr YouTube Cl + Kbr Ionic Equation enter an equation of an ionic chemical equation and press the balance button. Cl 2 (aq) + 2ki (aq). 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. a complete ionic equation is a chemical equation. Cl + Kbr Ionic Equation.
From www.chegg.com
Solved Classify each chemical reaction reaction KBr(aq) + Cl + Kbr Ionic Equation a solution of chlorine can displace iodine from potassium iodide solution: complete ionic equations show dissolved ionic solids as separated ions. a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. The balanced equation will be calculated along. write net ionic equations for chemical reactions between ionic compounds.. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVEDIn the ionic compounds LiF, NaCl, KBr, and RbI, the measured Cl + Kbr Ionic Equation Cl 2 (aq) + 2ki (aq). 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED Write the balanced COMPLETE ionic equation for the reaction Cl + Kbr Ionic Equation write net ionic equations for chemical reactions between ionic compounds. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. complete ionic equations show dissolved ionic solids as separated ions. Net ionic equations show only the. Chlorine. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED Balanced Equations Reactant(s) Yields H2O + KBr(s) Process and Cl + Kbr Ionic Equation 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. . Cl + Kbr Ionic Equation.
From dokumen.tips
(PDF) Formulas for Ionic Compounds formula unit KBr Ionic Naming.pdf Cl + Kbr Ionic Equation complete ionic equations show dissolved ionic solids as separated ions. Net ionic equations show only the. The balanced equation will be calculated along. Chlorine + potassium iodide → potassium chloride + iodine. enter an equation of an ionic chemical equation and press the balance button. Kbr (s) → k + (aq) + br − (aq) not only do. Cl + Kbr Ionic Equation.
From www.tessshebaylo.com
What Is The Net Ionic Equation For Reaction Between Pb C2h3o2 2 Aq And Cl + Kbr Ionic Equation The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. complete ionic equations show dissolved ionic solids as separated ions. Cl 2 (aq) + 2ki (aq). write net ionic equations for chemical reactions between ionic compounds. Net ionic equations show only the. Chlorine + potassium iodide →. Cl + Kbr Ionic Equation.
From www.youtube.com
Type of Reaction for KBr + Cl2 = KCl + Br2 YouTube Cl + Kbr Ionic Equation write net ionic equations for chemical reactions between ionic compounds. complete ionic equations show dissolved ionic solids as separated ions. The balanced equation will be calculated along. a solution of chlorine can displace iodine from potassium iodide solution: Net ionic equations show only the. Chlorine + potassium iodide → potassium chloride + iodine. Kbr (s) → k. Cl + Kbr Ionic Equation.
From www.scribd.com
Criss Cross Method for Predicting Formulas Chloride Ion Cl + Kbr Ionic Equation 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. enter an equation of an ionic chemical equation and press the balance button. a solution of chlorine can displace iodine from potassium iodide solution: a complete. Cl + Kbr Ionic Equation.
From gbu-presnenskij.ru
What Is The Ionic Equation For The Reaction Of Chlorine, 59 OFF Cl + Kbr Ionic Equation Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. Chlorine + potassium iodide → potassium chloride + iodine. a solution of. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED The following chemical reaction takes place in aqueous solution Cl + Kbr Ionic Equation Cl 2 (aq) + 2ki (aq). Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. Net ionic equations show only the. write net ionic equations for chemical reactions between ionic compounds. complete ionic equations show dissolved ionic solids as. Cl + Kbr Ionic Equation.
From www.slideserve.com
PPT Precipitate Reactions PowerPoint Presentation, free download ID Cl + Kbr Ionic Equation Cl 2 (aq) + 2ki (aq). a solution of chlorine can displace iodine from potassium iodide solution: a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. enter an equation of an ionic chemical equation and press the balance button. Net ionic equations show only the. write net. Cl + Kbr Ionic Equation.
From www.youtube.com
How to Balance KBr + Cl2 = KCl + Br2 (Potassium bromide + Chlorine gas Cl + Kbr Ionic Equation a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. write net ionic equations for chemical reactions between ionic compounds. Net ionic equations show only the. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED 3. Write balanced, net ionic equations for the reactions shown Cl + Kbr Ionic Equation complete ionic equations show dissolved ionic solids as separated ions. write net ionic equations for chemical reactions between ionic compounds. Cl 2 (aq) + 2ki (aq). The balanced equation will be calculated along. a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. 1 cl 2 + 1. Cl + Kbr Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for NaCl + KBr = NaBr + KCl YouTube Cl + Kbr Ionic Equation Net ionic equations show only the. write net ionic equations for chemical reactions between ionic compounds. Chlorine + potassium iodide → potassium chloride + iodine. a solution of chlorine can displace iodine from potassium iodide solution: complete ionic equations show dissolved ionic solids as separated ions. Kbr (s) → k + (aq) + br − (aq) not. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED In the reaction KBr + NH4Cl = NH4Br + KCl I have the complete Cl + Kbr Ionic Equation The balanced equation will be calculated along. 1 cl 2 + 1 kbr = 1 kcl + 1 br for each element, we check if the number of atoms is balanced on both sides of the equation. write net ionic equations for chemical reactions between ionic compounds. a solution of chlorine can displace iodine from potassium iodide. Cl + Kbr Ionic Equation.
From slidetodoc.com
Atomic Mass Unit amu atomic mass unit amu Cl + Kbr Ionic Equation enter an equation of an ionic chemical equation and press the balance button. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. complete ionic equations show dissolved ionic solids as separated ions. The balanced equation will be calculated along.. Cl + Kbr Ionic Equation.
From www.tessshebaylo.com
What Is The Net Ionic Equation For Reaction Between Pb C2h3o2 2 Aq And Cl + Kbr Ionic Equation complete ionic equations show dissolved ionic solids as separated ions. write net ionic equations for chemical reactions between ionic compounds. enter an equation of an ionic chemical equation and press the balance button. a solution of chlorine can displace iodine from potassium iodide solution: a complete ionic equation is a chemical equation in which the. Cl + Kbr Ionic Equation.
From www.numerade.com
SOLVED Consider the neutralization reaction between potassium Cl + Kbr Ionic Equation Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as. a solution of chlorine can displace iodine from potassium iodide solution: a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. . Cl + Kbr Ionic Equation.
From slideplayer.com
Acids and Bases L. ppt download Cl + Kbr Ionic Equation Chlorine + potassium iodide → potassium chloride + iodine. a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. a solution of chlorine can displace iodine from potassium iodide. Cl + Kbr Ionic Equation.